Abstract
Platelets are megakaryocyte subfragments that participate in hemostatic and host defense reactions and deliver pro- and antiangiogenic factors throughout the vascular system. Although they are anucleated cells that lack a complex secretory apparatus with distinct Golgi/endoplasmic reticulum compartments, past studies have shown that platelets have glycosyltransferase activities. In the present study, we show that members of 3 distinct glycosyltransferase families are found within and on the surface of platelets. Immunocytology and flow cytometry results indicated that megakaryocytes package these Golgi-derived glycosyltransferases into vesicles that are sent via proplatelets to nascent platelets, where they accumulate. These glycosyltransferases are active, and intact platelets glycosylate large exogenous substrates. Furthermore, we show that activation of platelets results in the release of soluble glycosyltransferase activities and that platelets contain sufficient levels of sugar nucleotides for detection of glycosylation of exogenously added substrates. Therefore, the results of the present study show that blood platelets are a rich source of both glycosyltransferases and donor sugar substrates that can be released to function in the extracellular space. This platelet-glycosylation machinery offers a pathway to a simple glycoengineering strategy improving storage of platelets and may serve hitherto unknown biologic functions.
Introduction
Glycosylation of proteins and lipids has a wide range of biologic functions.1 The glycosylation apparatus of nucleated cells is primarily located in the secretory pathway throughout the endoplasmic reticulum–Golgi stacks and consists of more than 200 glycosyltransferases, most of which are type II transmembrane-anchored proteins with distinct localization. Their topology within these compartments is directed by signal motifs contained in their stem, transmembrane, and cytoplasmic domains.2,3 Golgi-located membrane-bound glycosyltransferases are believed to be retained in the appropriate compartments by coat protein I–mediated retrograde transport3 and to be released and secreted only after proteolytic cleavage in their stalk regions to yield soluble, catalytically active truncated enzymes lacking their N-terminal transmembrane segment.4 Several previous studies have described unusual subcellular localization of glycosyltransferases outside of their normal confinement to the endoplasmic reticulum–Golgi (referred to herein as ectopic localization). Methodologic ambiguities, however, have cast doubt on these findings.5
One notable exception concerns reports of glycosyltransferase activities associated with blood platelets.6-11 Platelets are anucleated megakaryocyte subfragments that participate in hemostatic, inflammatory, and host defense reactions.12 Moreover, platelets deliver pro- and antiangiogenic factors throughout the vascular system.12 Glycosyltransferase activities in platelets were described 30 years ago and are proposed to mediate different aspects of platelet functions.6,7,13,14 These findings were surprising because platelets lack an organized glycosylation apparatus, and conclusive information is sparse regarding the biologic function of glycosyltransferases in platelets. We provided evidence previously that platelets have a surface-associated β4-galactosyltransferase 1 (β4Gal-T1) that couples galactose in a β1,4 linkage to N-acetylglucosaminyl (GlcNAc) bearing glycans on the GPIbα subunit of the VWF receptor, a process that improves the circulation of refrigerated platelets.11,15
In the present study, we characterized the expression and biogenesis of several classes of human glycosyltransferases in platelets using highly specific mAbs, enzyme isoform–specific functional assays, and in vitro thrombogenesis assays. We demonstrate that Golgi elements with glycosyltransferases are packaged into vesicles and transported from the megakaryocyte cell body to the nascent platelets, where they distribute intracellularly and to the plasma membrane. In addition, we show that activation of platelets releases substantial glycosyltransferase activity and promotes the incorporation of exogenously added FITC-conjugated cytidine monophosphate-sialic acid (FITC–CMP-SA) into plasma proteins in vivo, demonstrating that platelets are a rich source of glycosylation enzymes. Furthermore, our results indicate that platelets contain a sufficient pool of sugar nucleotides to support in vitro glycosylation reactions.
Methods
Animals
Age-, strain-, and sex-matched (male) C57BL/6 wild-type (WT), and α2,3-sialyltransferase IV knock-out mice (ST3GalIV−/−)16 (The Jackson Laboratory) were used in all experiments. Mice were maintained and treated as approved by the Harvard Medical Area Standing Committee on Animals according to National Institutes of Health standards as set forth in the Guide for the Care and Use of Laboratory Animals.
Platelet preparation
The preparation of human and mouse platelet-rich plasma was performed as described previously.17 After separation from plasma, platelets were washed in a solution of 140mM NaCl, 5mM KCl, 12mM trisodium citrate, 10mM glucose, 12.5mM sucrose, pH 6.0 (buffer A) and resuspended in 140mM NaCl, 3mM KCl, 0.5mM MgCl2, 5mM NaHCO3, 10mM glucose, and 10mM HEPES, pH 7.4 (buffer B). All centrifuge steps included prostaglandin E1 to prevent platelet activation. Approval for blood drawing was obtained from the institutional review board of Brigham and Women's Hospital, and informed consent was obtained according to the Declaration of Helsinki.
Glycosyltransferase assays
Polypeptide N-acetylgalactosaminyltransferase (GalNAc-transferase or GalNAc-T) and galactosyltransferase (Gal-transferase or Gal-T) assays, including sensitivity to lactalbumin, were performed as described previously.18-20 Sialyltransferase (sialyl-T or ST) assays were performed in reaction mixtures containing MES (pH 6.5), 20mM EDTA, 2mM DTT, 200-400μM CMP-[14C]-SA, and asialofetuin as an acceptor substrate with fetuin as a control. All experiments were repeated at least in triplicate. For details, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunoblot analysis
Platelets were lysed and proteins separated by SDS-PAGE were transferred onto an Immobilon-P membrane (Millipore). To detect the presence of glycosyltransferases, membranes were probed with mouse mAbs to human GalNAc-T1-3, β4Gal-T1, and ST3-Gal I,11,21 followed by peroxidase-tagged secondary Abs and detection with an ECL system (Pierce).
Flow cytometric analysis of platelet glycosyltransferases
Washed platelets were fixed with and without permeabilization in Cytofix or Cytofix/Cytoperm (BD Biosciences) and incubated with mouse mAbs to human GalNAc-T1-3, β4Gal-T1, and ST3-Gal I11,21 or with an isotype-specific irrelevant Ab control (DAKO), followed by incubation with Alexa Fluor 488 rabbit anti–mouse Fab, and flow cytometry (FACSCalibur; BD Biosciences). A total of 20 000 events were analyzed using BD CellQuest Pro software (BD Biosciences). All experiments were repeated at least in triplicate.
Platelet and megakaryocyte immunofluorescence microscopy
Resting platelets and megakaryocytes were immunostained as described previously22 with anti–human GalNAc-T1-3, β4Gal-T1, ST3-Gal I, and Golgi marker GM130 mAbs (R&D Systems), polyclonal anti-giantin (Abcam; a Golgi marker), anti-calnexin (Abcam), anti–P-selectin (R&D Systems), anti-VWF (DAKO), anti-serotonin (YC5/45; Abcam), and anti–γ-tubulin (Pierce). An isotype-specific irrelevant Ab was used as control. Experiments were repeated 4 times or more. To obtain murine megakaryocytes, livers were recovered from mouse fetuses at embryonic day 13, and single-cell suspensions were generated, immunostained, and analyzed as described previously.22 Sections of fixed murine megakaryocytes were stained and analyzed by electron microscopy as described previously.23
Live-cell imaging of GalNAc-T2-stem-YFP–infected megakaryocytes
Retroviral-mediated gene delivery was used to express GalNAc-T2-stem-yellow fluorescent protein (YFP) in mouse megakaryocytes using the pWZL retroviral vector.22 GalNAc-T2-YFP was constructed by fusing the stalk region of GalNAc-T2 to the NH2 terminus of YFP similar to production of the GalNAc-T2-green fluorescent protein described previously.24 A fragment encoding the cytoplasmic, transmembrane, and stalk regions was generated from the full-length GalNAc-T2 cDNA (accession number X85019) corresponding to amino acids 1-114 of the GalNAc-T2 protein. GalNAc-T2-stem-YFP movements were visualized by fluorescence microscopy 8-48 hours after infection. Infected megakaryocytes were transferred to video chambers maintained at 37°C and recorded as described in Patel et al22 and in supplemental Methods. Experiments were repeated at least 2 times.
Surface-active galactosyltransferase on platelets
Dynabeads M-270 Streptavidin (Invitrogen) were coupled to GlcNAcβ1-4GlcNAcβ-polyacrylamide (PAA)–biotin or Galα1-2Galβ-PAA-biotin (negative control; GlycoTech) for use as galactosyltransferase acceptor substrates. Assays were carried out using 0.4mM UDP-14C-Gal as a donor substrate and isolated and washed platelets (1 × 109/mL) as an enzyme source, in buffer A at 37°C for 2 hours. Recombinant β4Gal-T1 was used as the internal positive control.20 After incubation, the glycan-coupled Dynabeads were isolated and incorporated [14C]-Gal measured by scintillation counting. Release of soluble transferase from platelets during the incubation period was negligible as estimated by incubation of GlcNAcβ1-4GlcNAcβ-PAA-biotin and Galα1-2Galβ-PAA-biotin-Dynabeads with incubation buffer from the experiment after removing intact platelets.
Incorporation of sialic acid into human platelets
Isolated human platelets (109/mL) were activated using 25μM thrombin receptor–activating peptide (TRAP) for 5 minutes at 37°C or left untreated. After activation, untreated or activated platelets were adjusted to a concentration of 108/mL, incubated with 30μM FITC–CMP-SA or FITC as a control for 60 minutes at 37°C, and analyzed by flow cytometry or subjected to immunoblotting. For the identification of sialylated proteins and glycosylation status, glycosylated platelets were subjected to immunoblotting and lectin blotting as described in supplemental Methods.
Glycosyltransferase release from activated platelets
One milliliter of isolated human platelets (109/mL) was activated by 25μM TRAP for 5-10 minutes, centrifuged at 1000g, and GalNAc-T, Gal-T, and sialyl-T transferase activity measured (as described in “Glycosyltransferase assays”) in the pelleted material and in supernatant subjected to an additional ultracentrifugation step at 100 000g. Platelets were analyzed by flow cytometry before and after activation to confirm shape change and P-selectin exposure. In selected experiments, Gal-T activity was used as a model to test the release of enzyme after activation with 3, 6, 12.5, or 25μM TRAP or 6, 12.5, or 25μM ADP. Full activation and enzyme secretion were seen already at 5μM TRAP and 12μM ADP (not shown). Furthermore, inhibition studies were performed with washed, resting platelets incubated with 100μM acetylsalicylic acid or 10μM prostacyclin 5 minutes before activation with 25μM ADP and 25μM TRAP for 5 minutes, followed by measurement of secreted galactosyltransferase activity.
Presence of endogenous donor substrates
Washed human platelets (1 × 109) were incubated at 37°C for 4 hours in a 50- to 100-μL glycosylation assay mixture (25mM cacodylate, pH 7.4, 0.1% Triton X-100, 10mM MnCl2, EDTA-free protease inhibitor cocktail; Roche) and 300μM tetramethylrhodamine (TMR)–labeled βGlcNAc (TMR-βGlcNAc) or TMR-PEG-MUC1 (TMR-PEG-MUC1; with the MUC1 amino acid sequence AHGVTSAPDTRPAPGSTAPP). In one set of experiments, donor substrates (UDP-Gal and UDP-GalNAc) were included at concentrations of 0, 1.25, 5, or 20μM. Parallel platelet and Chinese hamster ovary cell lysates derived from identical packed cell volumes were incubated with 50μM concentrations of the acceptor substrates TMR-βGlcNAc or TMR-PEG-MUC1. Samples were analyzed by matrix-assisted laser desorption/ionization–time of flight mass spectrometry on a Voyager-DETM Pro Biospectrometry Workstation (Applied Biosystems) in positive linear or reflection mode. A separate set of experiments used secretions from platelets as the enzyme and donor sources. Secretions were obtained from TRAP-activated platelets as described in the preceding paragraph. Experiments were repeated 3-5 times.
Quantification of sugar nucleotides
Nucleotide sugars were extracted and analyzed by ion-pair HPLC analysis as described by Nakajima et al25 and in supplemental Methods.
Platelet glycosylation assays
For mouse and human platelet coincubation studies, 108 isolated human platelets and murine ST3GalIV−/− platelets were resuspended separately in buffer B. Human platelets were activated with 25μM TRAP for 5 minutes at 37°C in the presence or absence of externally added 30μM CMP-SA. After activation, human and murine ST3GalIV−/− platelets were mixed and incubated with PE-conjugated mouse Ab to human CD61 mAb (BD Biosciences) for 15 minutes at room temperature to identify human platelets. Surface glycan exposure was analyzed by incubation with FITC-conjugated RCA-I or Erythrina cristagalli lectin (0.1 μg/mL) specific for β-galactose. Mouse platelets negative for PE fluorescence were gated, and a total of 20 000 events were acquired and analyzed using BD CellQuest Pro software (BD Biosciences). For glycosylation studies using human platelet supernatants as the donor/substrate source, platelet supernatants were collected immediately after activation of 1010/mL of isolated human platelets with 25μM TRAP for 10 minutes at 37°C and ultracentrifugation at 100 000g for 30 minutes at 4°C to eliminate microparticles. A total of 100 μL of supernatants from activated or untreated platelets was added to 107 ST3GalIV−/− platelets and incubated for 60 minutes at 37°C. After the reaction, glycosylated platelets were transfused into WT mice or were lysed and subjected to immunoblotting as described in the next paragraph. Experiments were repeated 3 times.
Transfusion experiments
Glycosylated ST3GalIV−/− or untreated ST3GalIV+/+ platelets were loaded with 2.5μM green 5-chloromethylfluorescein diacetate (Invitrogen) for 15 minutes at 37°C. Unincorporated dye was removed by centrifugation at 100g for 5 minutes. 5-Chloromethylfluorescein diacetate–labeled platelets were injected into WT recipient mice via the retroorbital plexus, blood samples were collected at the indicated time points, and recovery and survival were measured as described previously.15 Platelet recovery was defined by the number of platelets circulating at the first sample point after injection, expressed as the percentage of circulating nonactivated WT platelets.
FITC-CMP-SA incorporation in vivo
A total of 40μM (final concentration) of FITC (AnaSpec) or FITC-CMP-SA (Boehringer-Mannheim) was injected into 3- to 4-week-old WT mice. Peripheral blood was obtained at 0, 10, 60, 120, and 180 minutes by retroorbital eye plexus bleeding. Platelet-rich plasma was obtained as described under “Platelet preparation,” activated with 25μM of TRAP for 5 minutes at 37°C, and analyzed immediately by flow cytometry (FACSCalibur). P-selectin expression was determined by flow cytometry using a PE-labeled mouse P-selectin mAb. The bleeding time was measured 60 minutes after injection by severing a 3-mm segment of a mouse tail. The amputated tail was immersed in 100 μL of saline at 37°C containing 0.1 volume of Aster-Jandl anticoagulant, and shed blood was collected and fixed in 1% paraformaldehyde. Peripheral blood was obtained by retro-orbital eye plexus bleeding, diluted 1/10 with saline, and fixed in 1% paraformaldehyde. For analysis of platelet activation in vivo, shed or obtained blood was labeled with PE-murine CD61 mAb (rat Ab to mouse CD61; clones 2C9G2 and LUCA5, Emfret Analytics)17 and analyzed by flow cytometry. Platelets that incorporated FITC-CMP-SA were identified by the FITC label. Experiments were repeated 2 to 3 times.
Bleeding time assay
FITC (300μM; AnaSpec) or FITC–CMP-SA (Boehringer Mannheim) was injected into 3- to 4-week-old WT mice. Sixty minutes after infusion of FITC or FITC–CMP-SA, mouse tail bleeding times were determined as described above by snipping 5 mm of distal tail and immediately immersing the tail fragment in 37°C isotonic saline.26 Time to complete cessation of bleeding was defined as the bleeding time. Measurements exceeding 10 minutes were stopped by cauterization of the tail. Experiments were repeated 2-3 times.
Statistical analyses
All data are presented as means ± SEM unless otherwise indicated. All numeric data were analyzed for statistical significance by 1-way ANOVA with Bonferroni correction for multiple comparisons using Prism software (GraphPad). P < .05 was considered statistically significant.
Results
Human platelets contain distinct classes of glycosyltransferases
Glycosyltransferase activity and the subcellular localization of the 3 functionally important glycosyltransferase gene families GalNAc-Ts, Gal-Ts, and sialyl-Ts were probed using isoform-specific enzyme assays and mAbs (Figure 1A-C). Activity assays with GalNAc-T–selective acceptors19 revealed the presence of at least 3 GalNAc-T isoforms in platelets (Figure 1A). Similarly, assays with variable acceptor concentrations and for lactalbumin-inducible lactose synthase activity20 detected multiple β4Gal-T activities, including β4Gal-T1, β4Gal-T2, and/or β4Gal-T3, whereas sialyl-T activity was detected using asialofetuin as the acceptor substrate (Figure 1A). The presence of GalNAc-Ts, Gal-Ts, and sialyl-Ts in human platelets was verified by immunoblotting (Figure 1B), flow cytometry (Figure 1C), and immunofluorescence microscopy (Figure 1D). Flow cytometry of nonpermeabilized and permeabilized platelets labeled with mAbs showed that the glycosyltransferases primarily localized to internal stores (Figure 1C broken lines). However, each enzyme could also be observed on the surface, with 40%-50% (P < .01) of platelets expressing β4Gal-T1, 10%-15% expressing sialyl-T, and 10%-15% expressing GalNAc-T on their surfaces (Figure 1C solid lines). The distribution of intracellular Golgi components was investigated by immunofluorescence of platelets with mAbs directed toward 3 GalNAc-Ts (T1, T2, and T3), 2 β4Gal-Ts (T1 and T2), ST3Gal-I, and ST6GalNAc-I, and the Golgi matrix protein GM130 (Figure 1D). Multiple intracellular granule-like compartments were stained in resting platelets (Figure 1D), which surprisingly did not colocalize with α- or dense granules or endoplasmic reticulum and only partially colocalized with lysosomal markers (supplemental Figure 1).
Human platelets contain functional surface and intracellular glycosyltransferases. (A) GalNAc-transferase, galactosyltransferase, and sialyltransferase activities in platelets. Detergent-extracted platelets were incubated with UDP-14C-GalNAc, UDP-14C-Gal, or CMP-14C-sialic acid (SA) and acceptor substrates. See supplemental Methods for details on acceptor substrates. Shown are the means ± SD of 3 experiments. (B) Immunoblots of platelet lysates subjected to SDS-PAGE using mAbs specific for GalNAc-T1, GalNAc-T2, GalNAc-T3, β4Gal-T1, and ST3Gal-I glycosyltransferases. Asterisks indicate bands with molecular weights corresponding to the glycosyltransferases (n = 3). (C) Flow cytometry histograms of GalNAc-T1 (red), β4Gal-T1 (green), and ST3Gal-I (blue) expression in nonpermeabilized (solid line) and permeabilized (dotted line) resting platelets. Isotype control mAbs (black line; n = 3). (D) Galactosyltransferase and Golgi marker (GM130) distribution in resting platelets determined using anti-GM130 and anti–GalNAc-T1, β4Gal-T1, and ST3Gal-I mAbs (n > 5). Insets show platelets at a higher magnification. Scale bars indicate 5 μm.
Human platelets contain functional surface and intracellular glycosyltransferases. (A) GalNAc-transferase, galactosyltransferase, and sialyltransferase activities in platelets. Detergent-extracted platelets were incubated with UDP-14C-GalNAc, UDP-14C-Gal, or CMP-14C-sialic acid (SA) and acceptor substrates. See supplemental Methods for details on acceptor substrates. Shown are the means ± SD of 3 experiments. (B) Immunoblots of platelet lysates subjected to SDS-PAGE using mAbs specific for GalNAc-T1, GalNAc-T2, GalNAc-T3, β4Gal-T1, and ST3Gal-I glycosyltransferases. Asterisks indicate bands with molecular weights corresponding to the glycosyltransferases (n = 3). (C) Flow cytometry histograms of GalNAc-T1 (red), β4Gal-T1 (green), and ST3Gal-I (blue) expression in nonpermeabilized (solid line) and permeabilized (dotted line) resting platelets. Isotype control mAbs (black line; n = 3). (D) Galactosyltransferase and Golgi marker (GM130) distribution in resting platelets determined using anti-GM130 and anti–GalNAc-T1, β4Gal-T1, and ST3Gal-I mAbs (n > 5). Insets show platelets at a higher magnification. Scale bars indicate 5 μm.
Dynamics of glycosyltransferases and Golgi markers during megakaryocyte differentiation
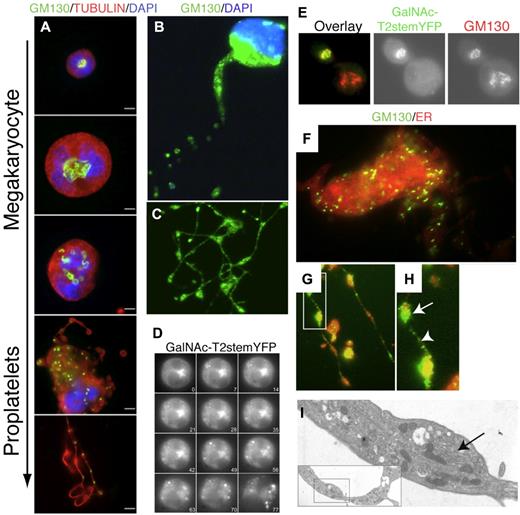
To investigate the origin of Golgi-derived glycosyltransferases in platelets, we followed the redistribution of Golgi markers during thrombopoiesis in vitro in murine megakaryocyte cultures. In this system, nascent platelets form at the ends of proplatelets elaborated by the megakaryocytes, where they are loaded with granules essential for hemostatic function.27 Because the mAbs used to visualize glycosyltransferases in human platelets do not react with murine enzymes, we used other Golgi markers to stain the megakaryocytes. First, murine megakaryocytes were stained at different maturation stages with a mAb recognizing the Golgi marker GM130 (Figure 2A-C)28 ; GM130 localized to the perinuclear area in immature megakaryocytes. However, just before proplatelets extended, GM130 was redistributed into cytoplasmic vesicular structures extending into proplatelets (Figure 2A-C).
Golgi redistribution during thrombogenesis. (A) Immunofluorescence micrographs of immature and proplatelet-producing megakaryocytes showing Golgi remodeling into condensed vesicles that move into proplatelets during megakaryocyte maturation. Tubulin is visualized with an antitubulin mAb (red); nuclei by DAPI staining (blue); Golgi by anti-GM130 Abs (green). The micrographs are representative of > 10 experiments. Scale bar indicates10 μm. (B-C) Location of GM130 in proplatelets. Golgi is fragmented and condensed into small vesicular structures (green). Scale bar indicates 10 μm. (D) Kinetics of the Golgi disassembly in a mouse megakaryocyte transfected with the Golgi marker GalNAc-T2-stem-YFP. Panels of micrographs selected from 77 minutes of real-time observation. (E) Double immunofluorescence microscopy of transfected megakaryocyte with GalNAc-T2-stem-YFP using anti-YFP (green) and anti-GM130 (red) Ab. Colocalization was seen in cells expressing GalNAc-T2-stem-YFP regardless of the maturation level (not shown). (F) Immunofluorescence microscopy of differentiated megakaryocytes using the Golgi marker anti-GM130 Ab (green) and the endoplasmic reticulum (ER) marker Calnexin (red). (G-H) Arrow shows GM130 accumulation in a proplatelet swelling. Between swellings, small GM130-containing vesicles are stained. (I) Electron micrograph of a section of a proplatelet extension/swelling containing a small Golgi-like vesicular stack (arrow).
Golgi redistribution during thrombogenesis. (A) Immunofluorescence micrographs of immature and proplatelet-producing megakaryocytes showing Golgi remodeling into condensed vesicles that move into proplatelets during megakaryocyte maturation. Tubulin is visualized with an antitubulin mAb (red); nuclei by DAPI staining (blue); Golgi by anti-GM130 Abs (green). The micrographs are representative of > 10 experiments. Scale bar indicates10 μm. (B-C) Location of GM130 in proplatelets. Golgi is fragmented and condensed into small vesicular structures (green). Scale bar indicates 10 μm. (D) Kinetics of the Golgi disassembly in a mouse megakaryocyte transfected with the Golgi marker GalNAc-T2-stem-YFP. Panels of micrographs selected from 77 minutes of real-time observation. (E) Double immunofluorescence microscopy of transfected megakaryocyte with GalNAc-T2-stem-YFP using anti-YFP (green) and anti-GM130 (red) Ab. Colocalization was seen in cells expressing GalNAc-T2-stem-YFP regardless of the maturation level (not shown). (F) Immunofluorescence microscopy of differentiated megakaryocytes using the Golgi marker anti-GM130 Ab (green) and the endoplasmic reticulum (ER) marker Calnexin (red). (G-H) Arrow shows GM130 accumulation in a proplatelet swelling. Between swellings, small GM130-containing vesicles are stained. (I) Electron micrograph of a section of a proplatelet extension/swelling containing a small Golgi-like vesicular stack (arrow).
To study the kinetics of the Golgi disassembly in vitro, we used a YFP-labeled chimeric expression construct of the Golgi-membrane anchor of human GalNAc-T2 (GalNAc-T2-stem-YFP) for tracking after transfection of megakaryocytes. The GalNAc-T2-stem-YFP signal localized in compact Golgi structures near the nuclei of immature megakaryocytes, but once proplatelets were extended, the GalNAc-T2-stem-YFP signal became scattered into cytoplasmic vesicular structures (Figure 2D and data not shown). GM130 and GalNAc-T2-stem-YFP always colocalized in transfected megakaryocytes (Figure 2E). Centrosomal disassembly, as evidenced by the dispersal of γ-tubulin staining, occurred before the Golgi disorganization, and intact centrosomes were not observed in megakaryocytes presenting a “disorganized” Golgi (supplemental Figure 2). In contrast, the endoplasmic reticulum remained diffusely distributed throughout the cytoplasm of megakaryocytes and proplatelets (Figure 2F-G). GM130 staining was noted in proplatelet swellings (Figure 2G-H), possibly reflecting Golgi-like organelles as observed by electron microscopy of proplatelet sections (Figure 2I arrow and inset).
Surface-exposed glycosyltransferases on resting platelets are functional
We also determined the functionality of surface-localized glycosyltransferase activity using 2 approaches: (1) acceptor substrates conjugated to Dynabeads that cannot be internalized because of their large size (2.3 μm in diameter) to probe β-galactosyltransferase activity (Figure 3A) and (2) FITC-labeled donor substrate (FITC-CMP-SA) to probe the incorporation of sialic acids into cell membrane glycoproteins by sialyltransferases directly (Figure 3B). Resting platelets catalyzed transfer of galactose from the donor UDP-14C-galactose to GlcNAcβ1-4GlcNAcβ-Dynabeads, whereas platelet-free medium did not (Figure 3A). No activity was seen with a control acceptor substrate GalNAcα1-3GalNAcα-Dynabead (Figure 3A), confirming that human platelets express functional surface β4Gal-T. We also incubated resting platelets with 30μM FITC-CMP-SA and monitored the incorporation of FITC-SA into surface glycoproteins by flow cytometry (Figure 3B) and SDS-PAGE immunoblotting with anti-FITC Ab (Figure 3C). A small but significant amount of FITC-SA incorporated into resting platelets, and increasing levels were detected after platelet PAR-1 activation with TRAP (Figure 3B). The major acceptor substrates identified by immunoblotting were the GPIbα subunit of the VWF receptor complex, the fibrinogen receptor subunit αIIb, and VWF (Figure 3C). These results are in agreement with our previous study showing that GPIbα carries glycans without sialic acid29 and reports of sialic acid incorporation into αIIb.30 We conclude that resting platelets express low levels of surface galactosyltransferases and sialyltransferases that can glycosylate extracellular glycoproteins.
Platelets glycosylate exogenous platelet and plasma acceptors. (A) Surface galactosyltransferase activity measured by incubating platelets with GlcNAc-βGlcNAc-PAA 2.8 μm (GlcNAcβ-R + Plt) or α-Gal-Dynabead conjugates (Galα-R + Plt) and UDP-14C-galactose. Galactosyltransferase activity released into the supernatant during incubation with Dynabead conjugates (GlcNAcβ-R + Sup and Galα-R + Sup). (B) Surface sialyltransferase incorporated FITC-conjugated CMP-SA (FITC-SA) into resting (dotted line) or TRAP-activated platelets. FITC alone (Control) was added to resting (dotted line) or TRAP-activated platelets (solid line). Quantification of resting and TRAP-activated platelet-associated mean fluorescence intensity (MFI); n = 3. (C) Immunoblots of lysates from resting (Rest) or TRAP-activated platelets (TRAP), treated with FITC (C) or CMP-FITC-SA (S) or left untreated (-) with Abs to FITC, GPIbα, αIIb, and VWF. Actin is shown as a loading control (n = 3).
Platelets glycosylate exogenous platelet and plasma acceptors. (A) Surface galactosyltransferase activity measured by incubating platelets with GlcNAc-βGlcNAc-PAA 2.8 μm (GlcNAcβ-R + Plt) or α-Gal-Dynabead conjugates (Galα-R + Plt) and UDP-14C-galactose. Galactosyltransferase activity released into the supernatant during incubation with Dynabead conjugates (GlcNAcβ-R + Sup and Galα-R + Sup). (B) Surface sialyltransferase incorporated FITC-conjugated CMP-SA (FITC-SA) into resting (dotted line) or TRAP-activated platelets. FITC alone (Control) was added to resting (dotted line) or TRAP-activated platelets (solid line). Quantification of resting and TRAP-activated platelet-associated mean fluorescence intensity (MFI); n = 3. (C) Immunoblots of lysates from resting (Rest) or TRAP-activated platelets (TRAP), treated with FITC (C) or CMP-FITC-SA (S) or left untreated (-) with Abs to FITC, GPIbα, αIIb, and VWF. Actin is shown as a loading control (n = 3).
Platelets serve as a reservoir of glycosyltransferases that are released during activation
Because we found that glycosyltransferases are predominately packaged inside platelets (Figure 1), we investigated the extent of transferase release after activation. Platelet activation through PAR-1 released approximately 50% of the total GalNAc-T, Gal-T, and sialyl-T glycosyltransferase activities to the soluble fraction of activated platelets (Figure 4). Using galactosyltransferase as a model enzyme, similar results were obtained by activating platelets with ADP. TRAP- and ADP-induced release of galactosyltransferase were inhibited by coincubation with acetylsalicylic acid and prostacyclin (supplemental Figure 4). Flow cytometry of activated platelets confirmed this activation and suggested that the platelets were still intact, demonstrating that the released enzyme was due to secretion rather than platelet lysis. Furthermore, the TRAP-induced release of enzyme activities was not microparticle associated because ultracentrifugation did not pellet the activity. Therefore, platelets contain and release a substantial number of glycosyltransferases. The release is presumably mediated by limited proteolysis of the stalk region, as demonstrated for many enzymes released from the Golgi compartments into the medium after activation.5
Activated platelets release soluble glycosyltransferases. GalNAc-, Gal-, and sialyltransferase activities in ultracentrifuged supernatant (S) from TRAP-activated platelets and detergent lysates of resting platelets (P). Glycosyltransferase activities were measured using C14-labeled donor sugars and enzyme-specific acceptor substrates (n = 3-4).
Activated platelets release soluble glycosyltransferases. GalNAc-, Gal-, and sialyltransferase activities in ultracentrifuged supernatant (S) from TRAP-activated platelets and detergent lysates of resting platelets (P). Glycosyltransferase activities were measured using C14-labeled donor sugars and enzyme-specific acceptor substrates (n = 3-4).
Platelets serve as a reservoir of activated sugar nucleotides
Because platelets secrete glycosyltransferases on activation and glycosylate glycoproteins on addition of exogenous sugar nucleotides, we investigated whether platelets contain and release sugar nucleotides. This capacity would imply that platelets have a “complete” glycosylation system for the modification of platelet, plasma, or endothelial glycoproteins. HPLC analysis by ion-pair reverse-phase chromatography (supplemental Figure 3) demonstrated that platelets contain substantial levels of UDP-GalNAc (418 pmol/mg), UDP-Gal (320 pmol/mg), UDP-Glc (1213 pmol/mg), UDP-GlcNAc (1195 pmol/mg), and CMP-SA (104 pmol/mg), which are only slightly below the levels found in nucleated cells.25 We also investigated whether the endogenous sugar nucleotide pool was sufficient to support detectable glycosylation on exogenously added acceptor substrates. Mass spectrometry of TMR-labeled acceptors was used to monitor the endogenous glycosylation capacity of total platelet lysates and soluble fraction of PAR-1–activated platelets (Figure 5). A TMR-MUC1 substrate was used to probe the donor UDP-GalNAc and TMR-βGlcNAc to probe the donor UDP-Gal because sufficient polypeptide GalNAc-T and β4Gal-T endogenous enzyme activity had already been demonstrated. Both platelet lysates and soluble fractions from secretions contained adequate amounts of donor sugar nucleotides for glycosylation with endogenous enzymes to produce TMR-MUC1-GalNAc and TMR-GlcNAc-Gal products (Figure 5). For comparison, preparations of total lysates from Chinese hamster ovary cells produced the same glycosylation products with the same efficacy (Figure 5).
Platelets glycosylate exogenous acceptors. Matrix-assisted laser desorption/ionization–time of flight mass spectrometry of glycosylation products in detergent extracts from resting platelets (Platelets), secretion from TRAP-activated platelets (Plt. secretion), Chinese hamster ovary cell lysates after addition of exogenous GalNAc-transferase acceptor TMR-PEG-MUC1 (left), or Gal-transferase acceptor TMR-βGlcNAc (right). No exogenous donor UDP-GalNAc or UDP-Gal was added, with the exception of the top panels, in which 100μM UDP-GalNAc and UDP-Gal were added, respectively (n = 3).
Platelets glycosylate exogenous acceptors. Matrix-assisted laser desorption/ionization–time of flight mass spectrometry of glycosylation products in detergent extracts from resting platelets (Platelets), secretion from TRAP-activated platelets (Plt. secretion), Chinese hamster ovary cell lysates after addition of exogenous GalNAc-transferase acceptor TMR-PEG-MUC1 (left), or Gal-transferase acceptor TMR-βGlcNAc (right). No exogenous donor UDP-GalNAc or UDP-Gal was added, with the exception of the top panels, in which 100μM UDP-GalNAc and UDP-Gal were added, respectively (n = 3).
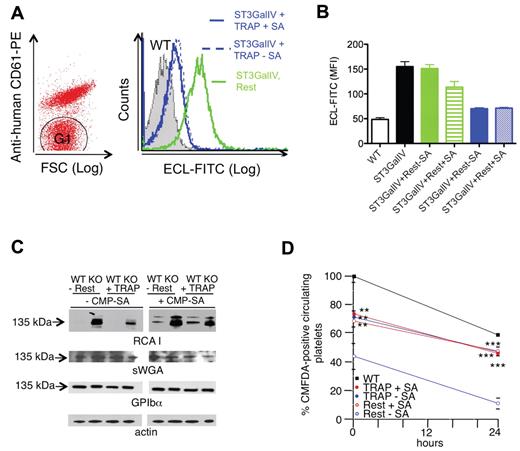
We also investigated whether the endogenous glycosylation machinery of platelets could sialylate the surface of other cells without the addition of exogenous sialyltransferase or CMP-SA donor substrate (Figure 6). Human platelets were mixed with platelets isolated from ST3Gal-IV−/− mice.31 ST3Gal-IV−/− platelet glycoproteins are terminated by β-galactose moieties, which strongly bind E cristagalli lectin.16 Therefore, sialic acid addition manifests as loss of E cristagalli lectin binding. Figure 6A-B compares the ability of resting and activated human platelets to sialylate ST3Gal-IV−/− platelets in the presence or absence of exogenous CMP-SA. The sialylation capacity of resting human platelets was low but enhanced by the addition of an exogenous CMP-SA donor. In contrast, selective activation of the human platelets through PAR-132 decreased E cristagalli lectin or RCA-I binding to ST3GalIV−/− mouse platelets by approximately 60% in the absence of added CMP-SA (Figure 6B-C). This result demonstrated the release of CMP-SA after platelet activation.
Platelets sialylate specific surface glycoproteins in neighboring cells. (A) Sialylation of GPIbα in ST3GalIV−/− mouse platelets by activated human platelets. Human platelets were identified and separated from mouse platelets (G1) by flow cytometry using PE-conjugated anti–human CD61. Flow cytometric analysis of terminal β-galactose using ECL-FITC-labeled lectin on mouse ST3GalIV−/− platelets (G1) incubated with TRAP-activated human platelets (TRAP) or resting platelets (Rest) in the absence of CMP-SA. Relative binding of ECL-FITC to ST3GalIV+/+ (WT) and ST3GalIV−/− (KO) platelets, and to ST3GalIV−/− platelets incubated with medium from resting (Rest) or TRAP-activated human platelets (TRAP) with (+SA) and without CMP-SA (-SA). (B) Histograms report the mean ± SEM. SA indicates CMP-SA (n = 3). (C) Incorporation of sialic acid in GPIbα. ST3GalIV+/+ (WT) and ST3GalIV−/− (KO) mouse platelets were incubated with supernatants from resting (Rest) or TRAP (TRAP)–activated human platelets with (+CMP-SA) or without (-CMP-SA) CMP-SA. Lysates were subjected to SDS-PAGE and immunoblotted with sWGA, RCA-I, or Abs to GPIbα and actin (n = 3). (D) Survival of ST3GalIV+/+ (WT) or ST3GalIV−/− mouse platelets incubated with resting (Rest) or TRAP-activated human platelet supernatants (TRAP) in the presence (+CMP-SA) or absence (-CMP-SA) of CMP-SA. The y-axis reflects the platelet recovery defined by the number of platelets circulating at the first sample point after injection, expressed as the percentage of circulating nonactivated WT platelets. Shown are means ± SEM for 6 recipients. **P < .01; ***P < .001.
Platelets sialylate specific surface glycoproteins in neighboring cells. (A) Sialylation of GPIbα in ST3GalIV−/− mouse platelets by activated human platelets. Human platelets were identified and separated from mouse platelets (G1) by flow cytometry using PE-conjugated anti–human CD61. Flow cytometric analysis of terminal β-galactose using ECL-FITC-labeled lectin on mouse ST3GalIV−/− platelets (G1) incubated with TRAP-activated human platelets (TRAP) or resting platelets (Rest) in the absence of CMP-SA. Relative binding of ECL-FITC to ST3GalIV+/+ (WT) and ST3GalIV−/− (KO) platelets, and to ST3GalIV−/− platelets incubated with medium from resting (Rest) or TRAP-activated human platelets (TRAP) with (+SA) and without CMP-SA (-SA). (B) Histograms report the mean ± SEM. SA indicates CMP-SA (n = 3). (C) Incorporation of sialic acid in GPIbα. ST3GalIV+/+ (WT) and ST3GalIV−/− (KO) mouse platelets were incubated with supernatants from resting (Rest) or TRAP (TRAP)–activated human platelets with (+CMP-SA) or without (-CMP-SA) CMP-SA. Lysates were subjected to SDS-PAGE and immunoblotted with sWGA, RCA-I, or Abs to GPIbα and actin (n = 3). (D) Survival of ST3GalIV+/+ (WT) or ST3GalIV−/− mouse platelets incubated with resting (Rest) or TRAP-activated human platelet supernatants (TRAP) in the presence (+CMP-SA) or absence (-CMP-SA) of CMP-SA. The y-axis reflects the platelet recovery defined by the number of platelets circulating at the first sample point after injection, expressed as the percentage of circulating nonactivated WT platelets. Shown are means ± SEM for 6 recipients. **P < .01; ***P < .001.
Sialylation diminished RCA-I (β-galactose exposure), binding primarily to the 135-kDa GPIbα polypeptide (Figure 6C), which is consistent with our previous findings that GPIbα is highly sialylated on ST3Gal-IV−/− platelets.16 ST3Gal-IV−/− platelets exhibit decreased recovery and survival after transfusion into WT mice because their sialic acid–deficient GPIbα is recognized by asialoglycoprotein receptors.16,29 Sialylation of GPIbα is predicted to improve the in vivo recovery and survival of transfused ST3Gal-IV−/− platelets. As a functional assay for the sialylation process, we analyzed the in vivo recovery and survival of murine ST3Gal-IV−/− platelets glycosylated by the secretion from PAR-1–activated human platelets. Glycosylation with secretion from human platelets improved ST3Gal-IV−/− platelet recovery and survival in mice significantly and was independent of exogenously added CMP-SA (Figure 6D). In contrast, exogenous CMP-SA was required when resting platelets were used in this assay (Figure 6D).
Incorporation of sialic acid on platelets decreases in vitro and in vivo activation
We next investigated whether sialyltransferases that modify surface-exposed glycans affect platelet function. FITC–CMP-SA (40μM) was mixed into mouse platelet-enriched plasma and the extent of FITC incorporation into the platelets was determined over time. FITC–CMP-SA incorporation into resting platelets was maximal within 1 hour (Figure 7A). Platelet activation through the mouse-specific thrombin receptor PAR-4 for 5 minutes in plasma increased the amount of FITC-SA incorporated by approximately 3-fold. This result suggests that FITC–CMP-SA was also incorporated into plasma proteins by activated platelets. However, the incorporation of sialic acid did not change initial platelet activation but slightly diminished activation after 60 minutes, as judged by shape change and up-regulation of P-selectin (Figure 7B-D). When FITC–CMP-SA was infused into mice to a final concentration of approximately 40μM (Figure 7E), CMP-SA–treated mice had approximately 60% longer bleeding times (380 ± 18 seconds) compared with mice infused with CMP alone (240 ± 63 seconds; P < .05). These results indicate that when CMP-SA is available in plasma, platelets incorporate sialic acid, making the platelets less responsive because of faster recovery to baseline after activation.
Sialic acid incorporation decreases platelet activation in vitro and in vivo. (A) Platelet-rich plasma from mice was incubated with FITC-conjugated CMP-SA or FITC (Control) at a 40μM final concentration for the indicated time points. At the indicated times, platelets in plasma were activated with 25μM TRAP for 5 minutes (TRAP) or left untreated (Rest), and platelet-associated FITC fluorescence was measured by flow cytometry (n = 2). (B) Representative side and forward scatter plots of platelets isolated from mice 60 minutes after infusion of FITC-SA or FITC (Control) and activated with 25μM TRAP or left untreated (Resting; n = 3). (C) The ratio of side to forward and side scatter platelets isolated from mice injected with FITC-SA (■) and FITC (□, Control) was determined. Unstimulated platelets isolated at 0 minutes were set as 1, and platelet shapes of isolates at 30 and 60 minutes activated with 25μM TRAP were compared. Data are means ± SD (n = 3). n.s. indicates not significant. *P < .05. (D) P-selectin expression was determined after TRAP activation by flow cytometry using a PE-conjugated anti–mouse P-selectin mAb (n = 2). (E) Activation status of platelets isolated from peripheral (Peripheral) or blood shed from a tail wound (Shed blood) 60 minutes after infusion of FITC (Control) or FITC-SA as assessed by light scatter characteristics. In the left panel, platelets are identified by PE–anti-CD61 mAb binding. The FITC mean fluorescence associated with platelets is plotted in the middle panel (n = 3; **P < .01. The right panel shows the ratio of side versus forward scatter of platelets in shed blood obtained from control mice (Control) or mice injected with FITC-SA (FITC-SA; n = 3; **P < .01).
Sialic acid incorporation decreases platelet activation in vitro and in vivo. (A) Platelet-rich plasma from mice was incubated with FITC-conjugated CMP-SA or FITC (Control) at a 40μM final concentration for the indicated time points. At the indicated times, platelets in plasma were activated with 25μM TRAP for 5 minutes (TRAP) or left untreated (Rest), and platelet-associated FITC fluorescence was measured by flow cytometry (n = 2). (B) Representative side and forward scatter plots of platelets isolated from mice 60 minutes after infusion of FITC-SA or FITC (Control) and activated with 25μM TRAP or left untreated (Resting; n = 3). (C) The ratio of side to forward and side scatter platelets isolated from mice injected with FITC-SA (■) and FITC (□, Control) was determined. Unstimulated platelets isolated at 0 minutes were set as 1, and platelet shapes of isolates at 30 and 60 minutes activated with 25μM TRAP were compared. Data are means ± SD (n = 3). n.s. indicates not significant. *P < .05. (D) P-selectin expression was determined after TRAP activation by flow cytometry using a PE-conjugated anti–mouse P-selectin mAb (n = 2). (E) Activation status of platelets isolated from peripheral (Peripheral) or blood shed from a tail wound (Shed blood) 60 minutes after infusion of FITC (Control) or FITC-SA as assessed by light scatter characteristics. In the left panel, platelets are identified by PE–anti-CD61 mAb binding. The FITC mean fluorescence associated with platelets is plotted in the middle panel (n = 3; **P < .01. The right panel shows the ratio of side versus forward scatter of platelets in shed blood obtained from control mice (Control) or mice injected with FITC-SA (FITC-SA; n = 3; **P < .01).
Discussion
The results of the present study demonstrate that megakaryocytes redistribute Golgi-derived glycosyltransferases into platelets, which accumulate in intracellular granules and at the platelet surface. We also show that platelets release a reservoir of glycosyltransferases and sugar nucleotides into the extracellular space on activation. Therefore, active soluble glycosyltransferases are secreted after platelet activation, which, along with their donor substrates, enables platelets to both initiate and elongate glycans on extracellular acceptor molecules. Although we currently take advantage of this ectopic function of glycosyltransferases in our glycoengineering approach to improving circulation of cold-stored platelets, future studies will be required to delineate the functional details of this extracellular glycosylation machinery.
Ectopic expression of glycosyltransferases outside the endoplasmic reticulum–Golgi has long been a controversial issue. The conclusions related to their expression were based on immunohistochemistry and the ability to incorporate radiolabeled sugars into surface proteins.5-7,33-35 Many of the studies, however, have been questioned because of the use of nonspecific polyclonal Abs and mAbs without well-characterized specificities. In particular, the use of nonspecific polyclonal Abs cross-reacting with either carbohydrates or irrelevant proteins has in several cases led to questionable conclusions (for review, see Berger5 ). Moreover, functional activity assays of putative cell-surface enzymes have not excluded unambiguously that the measured activity was a result of intracellular enzyme activity, such as cellular uptake of donor/acceptor substrates.36 With these reservations in mind, studies using affinity-purified polyclonal Abs have demonstrated ectopic glycosyltransferases in cancer cells and certain highly differentiated cells.5,37-40 In the present study, we show that intact platelets can glycosylate substrates bound to particles that cannot be endocytosed. Furthermore, we show that platelets express sialyltransferases that can sialylate surface proteins on neighboring cells, demonstrating that platelet surface glycosyltransferases have an ectopic function. These results establish unambiguously the presence of functional glycosyltransferases on the surface of platelets. Megakaryocytes and platelets are highly differentiated and specialized cells, and our findings are therefore consistent with previous studies on differentiated cells such as neurons and endothelial cells.38-40
In addition to our experiments demonstrating that glycosyltransferases are packaged inside platelets and can be delivered to the surface, expression studies of a YFP-tagged glycosyltransferase (GalNAc-T2) in cultured megakaryocytes allowed us to visualize the mechanism by which glycosyltransferases are transported into platelets. Platelets are released from megakaryocytes in a process that remodels the entire megakaryocyte cytoplasm into long, beaded extensions termed proplatelets.27 Platelet formation begins as microtubules are released from the centrosome and align into cortical bundles, which are subsequently bent to protrude the initial proplatelets. These microtubule bundles then elongate and run from the cell body to the end of the proplatelets, serving as both the motor for elongation and as tracks on which organelles move.27 We found that as megakaryocytes matured, YFP-labeled GalNAc-T2 and the Golgi marker GM130 redistributed from a centralized Golgi into numerous cytoplasmic vesicular structures that localized along the microtubule tracks of proplatelets. This observation suggests that glycosyltransferases are packaged into granules when proplatelets are produced and sent to the nascent platelets by motors that move along microtubule tracks. The result may be Golgi outposts that could ensure glycosylation of de novo translated glycoproteins in platelets in a way similar to that proposed for dendrites in neurons.41 Consistent with this speculation, platelets possess a functional RNA spliceosome that enables them to create mature message RNA and translate it into protein.42 This capacity may suggest that the presence of functional Golgi glycosyltransferases and donor sugars in platelets is related to requirements for posttranslational modification of newly expressed proteins.
Substantial evidence documents the presence of soluble active fragments of glycosyltransferases in plasma, including blood group AB enzymes and β4Gal-T activity, but the origin and function of these are unclear. Because plasma does not contain measurable sugar nucleotides, extrinsic glycosylation in the blood has been considered unlikely. In the present study, we found that platelets contain an amount of sugar nucleotides on a volume basis that is equivalent to the amount found in nucleated cells. Even if the reservoir of sugar nucleotides were released from a large proportion of platelets, the amount in the plasma would most likely be insufficient to allow for enzymatic activity of freely circulating glycosyltransferases. In contrast, the sugar nucleotide concentration could be sufficient at local sites of activation, where platelet aggregates limit diffusion.43 By this process, platelet glycosylation at the site of activation could be speculated to regulate receptor functions in the gap between aggregated platelets, enabling outside-in signaling during thrombus formation.43,44 In support of this speculation, we found herein that activated platelets sialylate both VWF and GPIb when incubated with fluorescently labeled CMP-SA (Figure 2C). Loss of α2-3–linked sialic acid significantly increases VWF binding to GPIbα, and platelets therefore deliver a more potent VWF to promote thrombus formation in vivo.16,45 Furthermore, loss of α2-6-linked sialic acid on VWF specifically enhances proteolysis by ADAMTS13.46 The identified sialylation could therefore explain the diminished activation level of sialylated platelets via an inhibitory function of sialic acid on VWF activation of GP1bα, suggesting a role in rejuvenation and platelet circulation rather than in thrombus formation.
The role of glycans in platelet circulation lifetime has been characterized previously. We showed in earlier studies that chilling of mouse platelets induces clustering of N-linked glycans located on GPIbα.11,29 This clustering exposes uncapped βGlcNAc and βGal termini and leads to lectin-mediated platelet clearance from the circulation by macrophage αMβ2 receptors and asialoglycoprotein receptors.29,47 The reorganization of the Golgi observed during thrombopoiesis provides a potential explanation for the coexistence of glycosyltransferases and incomplete glycans on platelet surfaces after platelet activation. With the aim of using glycoengineering to prevent clearance of cold-stored platelets, we have shown that addition of the donor substrate UDP-Gal causes glycosylation of exposed GlcNAc residues on GPIbα, preventing recognition of cold-stored platelets by the hepatic macrophage αMβ2 clearance system.11,15,48 The present study demonstrates that platelet sialyltransferases transfer fluorescently labeled CMP-SA to specific platelet surface proteins, capping exposed galactose and preventing the clearance of platelets mediated by the hepatic asialoglycoprotein receptor.29 These findings suggest that glycoengineering could prevent the recognition of cold-stored platelets using a combination of galactosylation and sialylation.48 Immature glycans could also represent essential clearance signals on activated circulating platelets, and it is tempting to speculate that reglycosylation of exposed immature glycans could prolong platelet survival. In support of this notion, desialylated GPIbα was shown previously to be a target for TACE (ADAM17), which inactivates VWF receptor function.49 The demonstrated ability of platelet secretions to increase the circulation time of desialylated human platelets provides additional experimental support.
The human glycome depends on a complex biosynthetic machinery involving the regulation of hundreds of glycosyltransferases, as well as the coordinated availability of donor sugar nucleotides and relevant acceptor substrates. The secretion of both enzymes and donor sugars from a novel granule structure in platelets enables such functions in a space devoid of the constraints of the Golgi apparatus. Future studies will aim to investigate the functional importance of the unique glycosylation apparatus found in platelets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sara Nayeb-Hashemi, Max Adelman, and Shu Young for excellent technical assistance. The acceptor substrates TMR-PEG-MUC1 and TMR-GlcNAc were a generous gift from Monica M. Palcic, Carlsberg Laboratory, Denmark.
This work was supported by the Danish Medical Research Council Award, The Lundbeck Foundation, Agnes and Poul Friis, The Carlsberg Foundation, and the Program of Excellence, Copenhagen Center of Glycomics, Faculty of Health Sciences, University of Copenhagen. Additional support was provided by the National Institutes of Health (grant HL56949 to K.M.H. and J.H.H., grants PO1HL107146 and HL089224 to K.H., a Pew Scholars Award to K.M.H., and HL68130 to J.I.).
National Institutes of Health
Authorship
Contribution: H.H.W. and K.M.H. designed the research, performed the experiments, and wrote the manuscript; V.R. designed and performed the experiments; A.L.T.S. performed the experiments and wrote the manuscript; S.P.-H. performed the experiments; E.C.J. and J.E.I. provided essential methodology and intellectual contribution; E.P.B. provided essential reagents; and H.C. and J.H.H. wrote the manuscript.
Conflict-of-interest disclosure: H.H.W., V.R., and K.M.H. received sponsored research support from ZymeQuest. K.M.H. is a consultant for Velico Inc (previously ZymeQuest Inc). The remaining authors declare no competing financial interests.
The current affiliation for E.C.J. is The Walter and Eliza Hall Institute, Cancer & Hematology Division, Parkville, Victoria, Australia.
Correspondence: Hans H. Wandall, Department of Cellular and Molecular Medicine, Faculty of Health Sciences, University of Copenhagen, Blegdamsvej 3, 2200 Copenhagen N, Denmark; e-mail: hhw@sund.ku.dk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal