Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia/lymphoma and HTLV-1–associated myelopathy/tropical spastic paraparesis. In addition to blood transfusion and sexual transmission, HTLV-1 is transmitted mainly through prolonged breastfeeding, and such infection represents a major risk for the development of adult T-cell leukemia/lymphoma. Although HTLV-1–infected lymphocytes can be retrieved from maternal milk, the mechanisms of HTLV-1 transmission through the digestive tract remain unknown. In the present study, we assessed HTLV-1 transport across the epithelial barrier using an in vitro model. Our results show that the integrity of the epithelial barrier was maintained during coculture with HTLV-1–infected lymphocytes, because neither morphological nor functional alterations of the cell monolayer were observed. Enterocytes were not susceptible to HTLV-1 infection, but free infectious HTLV-1 virions could cross the epithelial barrier via a transcytosis mechanism. Such virions were able to infect productively human dendritic cells located beneath the epithelial barrier. Our data indicate that HTLV-1 crosses the tight epithelial barrier without disruption or infection of the epithelium to further infect target cells such as dendritic cells. The present study provides the first data pertaining to the mode of HTLV-1 transport across a tight epithelial barrier, as can occur during mother-to-child HTLV-1 transmission during breastfeeding.
Introduction
Approximately 5-20 million persons worldwide are infected by the human retrovirus human T-cell leukemia virus type 1 (HTLV-1),1 and this virus is the causative agent of severe adult T-cell leukemia/lymphoma2 and inflammatory syndromes such as tropical spastic paraparesis/HTLV-1–associated myelopathy, a slowly progressing neurodegenerative disease that occurs in 0.5%-3% of infected persons.3 HTLV-1 is transmitted via sexual intercourse, by transfusion with contaminated blood, and from mother to child. The latter constitutes 15%-25% of overall transmission, so it is a major method of viral spread.4,5 Although intrauterine or perinatal transmission during birth cannot be excluded, breastfeeding is the main pathway of HTLV-1 transmission in highly endemic areas such as intertropical Africa, the Caribbean, or regions of South America.6,7 Milk-borne transmission is supported by the presence of high levels of infected lymphocytes in maternal milk, 105-107 of these being transferred to the child at each feed,8,9 in addition to infected macrophages and breast epithelial cells. Furthermore, leukocytes in the breast milk remain viable after ingestion for up to 4 hours because of the buffering capacity of breast milk and the low acidity of the neonatal child's stomach.10,11 The risk of transmission increases with the time that children are breastfed, especially when this exceeds 6 months. In addition to the duration of breastfeeding, the mother's age, Ab titers directed against HTLV-1 antigens, proviral load in the PBMCs and milk, and the concordance of the HLA class I type between mother and child are all considered to play roles in mother-to-child transmission of HTLV-1.12 Virus uptake on breastfeeding may occur in the tonsil mucosa (pluristratified epithelium) or intestinal mucosa (monostratified epithelium) of the infant or at both of these sites.
The penetration of mucosal surfaces as early steps of infection has been studied extensively for retroviruses such as HIV. In the case of a monostratified epithelium, it was found that HIV particles bud from PBMCs, bind to the apical membrane of epithelial cells, are internalized, and appear on the basolateral side of the intestinal cells as early as 30 minutes later, clearly having used the apical-to-basolateral transcytotic pathway.13-15 Many previous studies on the passage of pathogens across the intestinal barrier relied on the use of intestinal epithelial cell lines cultured as monolayers on permeable filters to align the apical and basolateral sides of the cells. Such studies showed that, in addition to direct infection of epithelial cells, which is known for rotaviruses,16,17 other mechanisms also exist. For example, specialized M-cells contained in the follicular-associated epithelium in the gut can serve as the portal entry for polioviruses or prions.18,19 In addition, it has been shown that even dendritic cells (DCs) located in the subepithelial dome and the lamina propria directly underneath the epithelial barrier can be actively involved in the uptake of bacteria.20
To date, the passage of HTLV-1 across the digestive tract has not been studied thoroughly. It is known that oral administration of this virus via infected lymphocytes can lead to seroconversion in animals.21 Except for a single in vitro study devoted to epithelial cell infection by HTLV-1,22 no attention has been paid to HTLV-1 interactions and passage across a tight enterocyte monolayer. It is known that DCs are susceptible to HTLV-1 infection by both cell-associated23-25 and cell-free viruses26,27 in vitro as well as in vivo and that they are able to transmit the infection to CD4 T cells, the main in vivo target of HTLV-1.
The present study elucidates the early steps of transmucosal penetration of HTLV-1 into a tight cellular monolayer. Using an in vitro model of human intestinal cells grown on filters and cocultured with HTLV-1–infected lymphocytes, we found that barrier integrity was not compromised in either morphology or function. Three different intestinal cell lines were found to be insensitive to productive HTLV-1 infection, but HTLV-1 virions penetrated the epithelial barrier by transcytosis. Furthermore, we demonstrate herein that such a transcytosed virus was able to infect DCs located immediately below the epithelial monolayer.
Methods
Cell culture and growth conditions
Intestinal cells.
The Caco-2 human epithelial cell line (ATCC) derived from an adenocarcinoma was grown as described previously.28 Briefly, cells were grown in DMEM, 4.5 g/L of glucose (Gibco), 10% FCS (Gibco), and 1% nonessential amino acids (Gibco). Cells were seeded either on 13-mm-diameter glass slides or on polycarbonate filters (12-mm diameter, 0.45-μM pore size; Costar). Cells were seeded at a density of 0.5-1 × 105 cells per filter and grown for 14 days at 37°C in 5% CO2 with a change of medium every other day.
In some experiments, 2 other enterocytic cell lines, HT29 and T84, were used. HT29 cells, also derived from a human adenocarcinoma, were grown under exactly the same culture conditions as Caco-2 cells. The T84 cell line, which originates from a human colon carcinoma, was cultured in F12 medium (Gibco) supplemented with 10% FCS (Gibco) at 37°C in 5% CO2.
Lymphoid cells.
The infected C91-PL cell line was used as a source of HTLV-1. This cell line is derived from human umbilical cord blood T cells transformed by HTLV-1.29 CEM, an HTLV-1− T-cell line derived from an acute lymphoblastic leukemia, was used as a negative control. The cells were grown in RPMI 1640 medium (Gibco) containing 1mM glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% FCS (Gibco).
DCs.
Human monocyte-derived DCs were prepared and maintained as described previously30 in RPMI medium containing 10% FCS and enriched with IL-13 (Sanofi-Synthelabo) and GM-CSF (Novartis) cytokines.
HEK 293-LTR-GFP cells.
Human embryonic kidney (HEK) 293 epithelial cells were grown at 37°C in 5% CO2, in DMEM (Invitrogen) containing 1mM glutamine, 100 U/mL of penicillin and streptomycin, and 10% FCS (Gibco). These cells contain an integrated HTLV-1 long-terminal repeat (LTR) coupled to a green fluorescent protein (GFP) reporter gene and are therefore called 293-LTR-GFP.
Cell coculture
The day before the onset of coculture, lymphocytes were seeded at 400 000 cells/mL, which was adjusted to 1 × 106 cells/mL on the day of the experiment. They were finally added onto the apical pole of Caco-2 cells (apical chamber) at a 1:1 ratio. To determine the viral protein content in the basolateral chamber, the medium was either ultracentrifuged at 64 000g for 2 hours at 4°C and the pellet was resuspended in fresh medium and subjected to SDS-PAGE and Western blot designed to detect the p24 protein or it was analyzed directly by ELISA to determine the amount of p19 protein (see “Transcytosis assay”).
To test the susceptibility of different epithelial cell lines to HTLV-1 infection, 5 days before the onset of the assay Caco-2 (2.5 × 104 cells/well), HT29 (2 × 104) and T84 cells (30 × 104) were seeded in 24-well plates containing glass coverslips.
Lymphocytes were seeded at 4 × 105 cells/mL the day before the start of the experiment, and were irradiated at a dose of 10 Gy (debit: 0.25 Gy/min). When cultures had reached approximately 85% confluence, 50 × 105 lymphocytes were added per well. Cells were fixed after 1, 3, 7, 14, or 21 days and were processed for immunofluorescence (see below). Production of the viral p19 protein in the medium was determined by ELISA.
For the incorporation of DCs into the coculture system, 3-μm pore size filters containing differentiated Caco-2 cells were turned upside down and a drop (80 μL) containing 1-1.5 × 106 DCs in DC medium was added. After 4 hours of incubation at 37°C, the Transwells were turned over again and C91-PL or CEM lymphocytes were added to the apical chamber. Four days after coculture, filters were either fixed with formalin solution (Sigma-Aldrich) for analysis by immunofluorescence (p24) or centrifuged at 200g to detach adherent DCs from the filter for HTLV-1 infection analysis.
Monolayer tightness and permeability assay
Tightness of the Caco-2 monolayer was monitored routinely by measuring transepithelial resistance (TER) using an epithelial volt-ohm meter (EVOM; World Precision Instruments). TER values ranged from 280-320 ohms/cm2 at Caco-2 cell total confluence. Paracellular permeability of the epithelium was monitored by measuring the transit of FITC-labeled dextran (molecular weight, 4 kDa; Molecular Probes). Culture medium was replaced by DMEM without phenol red. FITC-labeled dextran was added to the upper chamber and filters were sequentially transferred from well to well for 1 hour at 15-minute intervals. The quantity of dextran molecules that had diffused through the monolayer into the basolateral compartment was determined using a fluorescence plate reader (Wallac Victor 1420; Perkin Elmer). Treatment with EDTA, a calcium chelator known to open the barrier, was used as a positive control (12.5mM added 30 minutes before coculture with lymphocytes). Permeability coefficients of the epithelium were then calculated as described previously.31 The expression of tight junction proteins was checked 48 hours after cell-to-cell contact. The monolayer was fixed and labeled for occludin and zonula occludens-1 (ZO-1) using primary Abs (Zymed) and observed by fluorescence microscopy.
Transcytosis assay
Epithelial cells were grown as tight monolayers on 0.4-μm filters. HTLV-1–infected lymphoid cells were added to the apical chamber at a ratio of 1:1 (lymphoid to epithelial cells) in 500 μL of medium. The temperature dependence of virion passage across the monolayer was tested by incubating the coculture at either 37°C or 4°C. Analysis of the transcytotic viral particles was performed either by transmission electron microscopy, or by ELISA designed to measure the p19 in the basolateral compartment after 2 hours after contact (ELISA p19 kit; Zeptometrix). Infectivity of the viral particles harvested from the basolateral compartment was assessed on 293-LTR-GFP cells or by immunofluorescence for p24 as described previously31
Cryofixation and electron microscopy
For ultrastructural analysis at different times after coculture, cells were fixed (4% paraformaldehyde) and dehydrated in ethanol. Samples were embedded in Lowicryl HM20 resin at low temperature, and 70-nm thin sections were prepared for immunogold labeling of the viral p24 protein. Sections were incubated in PBS supplemented with 0.25% NH4Cl for 20 minutes and PBS with 1% BSA for 10 minutes. After 30 minutes of incubation with the primary Ab (anti-p24; Abcam), samples were washed and incubated for 10 minutes with the secondary Ab (Sigma-Aldrich) conjugated to colloidal gold (10-nm gold particles). Preparations were washed and contrasted with uranyl acetate. Samples were observed using a JEOL 1010 transmission electron microscope and images acquired with a CCD camera.
Immunofluorescence
Lymphocytes and DCs were stained with the living dye CellTracker 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen) before the onset of the experiment, following the manufacturer's recommendations.
After coculture, Transwell filters or coverslips were fixed with paraformaldehyde. Coverslips were preincubated for 30 minutes in normal horse serum (10%) before incubation with a primary mouse mAb directed against the HTLV-1 p24 capsid protein (Abcam). Secondary goat anti–mouse IgG 549-conjugated Abs were used (Pierce). The coverslips were mounted with DAPI-containing Vectashield (Vector Laboratories). Depending on the experiment, a Nikon Microphot-FXA, a Leica DMRB fluorescence microscopes, or a Zeiss Axiovert apparatus with the Zeiss ApoTome system was used for observation at the Imaging Platform of the Pasteur Institute.
Immunoblot analyses
Cells were washed once in cold PBS and incubated in lysis buffer (50mM Tris, pH 7.5, 150mM NaCl, 5mM EDTA, 1% NP-40, 0.1% SDS, and protease inhibitors [Roche]) on ice. Samples were loaded onto Bis-Tris gels (10% acrylamide; NuPage; Invitrogen), subjected to SDS-PAGE, and transferred onto a nitrocellulose membrane (Hybond; Amersham).
After incubation in the presence of the primary anti-p24 mouse Ab (Abcam) and extensive washing in PBS-Tween 0.1%, detection was performed with a peroxidase-conjugated anti–mouse secondary Ab (Amersham). Protein bands were revealed using the ECL kit (Amersham).
Results
Coculture of epithelial cells with infected lymphocytes does not alter epithelial barrier integrity
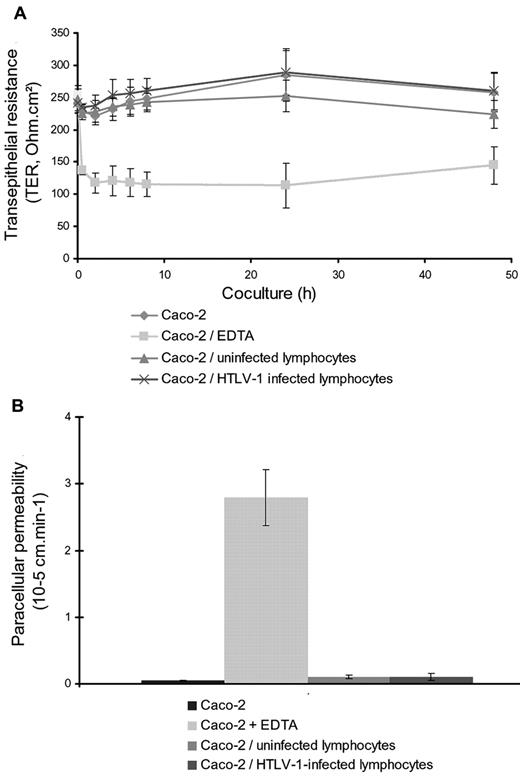
We first assessed the effects of HTLV-1–infected lymphocytes on the integrity of the epithelial barrier in vitro (Figure 1A). Electron and immunofluorescence microscopy observations indicated that coculture for 48 hours did not modify the morphological characteristics of the epithelial barrier. In particular, tight junctions between epithelial cells were still observed (Figure 1C), as seen in untreated controls or in cocultures of epithelial cells with uninfected CEM lymphocytes (Figure 1B). Similarly, localization of the tight junction proteins ZO-1 and occludin was not modified (Figure 1D-G). Moreover, the functional integrity of the epithelial barrier was conserved after coculture with infected lymphocytes: both TER and transport of small molecular markers (dextran, 4 kDa) between the apical and basal compartments were identical between cocultures of Caco-2 cells with infected or uninfected lymphocytes (Figure 2A-B, respectively), as well as for Caco-2 cells alone. Results were compared with EDTA-treated cocultures, which were used as a positive control to open the barrier that was shown to decrease the TER and to increase the paracellular permeability (Figure 2A-B, respectively)
Microscopic observations of tight junctions between Caco-2 cells cocultured with HTLV-1–infected or uninfected lymphocytes. (A) Experimental setting to study the viral passage after coculture. Caco-2 cells were cultured on Transwell filters before the addition of HTLV-1–infected (or uninfected) lymphocytes to the apical compartment. Two days later, cocultures were fixed and processed for observations by transmission electron microscopy or immunofluorescence microscopy. (B-C) Transmission electron microscopy showing tight junctions (indicated by black arrows) between epithelial cells cocultured with uninfected lymphocytes (B) and with HTLV-1–infected lymphocytes (C). Scale bar indicates 1 μm. Observation was with a JEOL 1010 transmission electron microscope operating at 80 kV. Image acquisition was with a Nikon CCD camera. (D-G) Detection of tight-junction proteins by immunofluorescence. Immunoreactivity for occludin and ZO-1 (D-E and F-G, respectively) could be detected according to the same pattern between epithelial cells cocultured with uninfected lymphocytes (D,F) or with HTLV-1–infected lymphocytes (E,G). Original magnification, 200×. Observation was with a Leica DMRB microscope equipped for fluorescence. Image acquisition was with a Nikon Coolpix 8400 camera.
Microscopic observations of tight junctions between Caco-2 cells cocultured with HTLV-1–infected or uninfected lymphocytes. (A) Experimental setting to study the viral passage after coculture. Caco-2 cells were cultured on Transwell filters before the addition of HTLV-1–infected (or uninfected) lymphocytes to the apical compartment. Two days later, cocultures were fixed and processed for observations by transmission electron microscopy or immunofluorescence microscopy. (B-C) Transmission electron microscopy showing tight junctions (indicated by black arrows) between epithelial cells cocultured with uninfected lymphocytes (B) and with HTLV-1–infected lymphocytes (C). Scale bar indicates 1 μm. Observation was with a JEOL 1010 transmission electron microscope operating at 80 kV. Image acquisition was with a Nikon CCD camera. (D-G) Detection of tight-junction proteins by immunofluorescence. Immunoreactivity for occludin and ZO-1 (D-E and F-G, respectively) could be detected according to the same pattern between epithelial cells cocultured with uninfected lymphocytes (D,F) or with HTLV-1–infected lymphocytes (E,G). Original magnification, 200×. Observation was with a Leica DMRB microscope equipped for fluorescence. Image acquisition was with a Nikon Coolpix 8400 camera.
Assessment of barrier integrity of Caco-2 monolayer cocultured with HTLV-1–infected or uninfected lymphocytes. Results shown are the means of 3 independent experiments. (A) Measurement of TER monitored with a volt-ohm meter at different times after coculture. Similar values of TER were found over time for Caco-2 cells cultured alone (diamonds) or with uninfected lymphocytes (triangles) or HTLV-1–infected lymphocytes (crosses) compared with the low values measured after EDTA treatment (squares). EDTA (12.5mM) was added 30 minutes before coculture with lymphocytes and used as a positive control to open the barrier. (B) Assessment of paracellular permeability of the Caco-2 monolayer monitored by FITC dextran (4 kDa) transfer to the basal compartment. No significant amounts of FITC dextran could be detected in the basal compartment for Caco-2 cells cultured alone (first column to the left) or with uninfected lymphocytes (third column to the left) or HTLV-1–infected lymphocytes (fourth column to the left). Opening of the barrier (positive control) was performed by adding EDTA in the apical medium, leading to a significant increase of permeability to FITC dextran (second column to the left).
Assessment of barrier integrity of Caco-2 monolayer cocultured with HTLV-1–infected or uninfected lymphocytes. Results shown are the means of 3 independent experiments. (A) Measurement of TER monitored with a volt-ohm meter at different times after coculture. Similar values of TER were found over time for Caco-2 cells cultured alone (diamonds) or with uninfected lymphocytes (triangles) or HTLV-1–infected lymphocytes (crosses) compared with the low values measured after EDTA treatment (squares). EDTA (12.5mM) was added 30 minutes before coculture with lymphocytes and used as a positive control to open the barrier. (B) Assessment of paracellular permeability of the Caco-2 monolayer monitored by FITC dextran (4 kDa) transfer to the basal compartment. No significant amounts of FITC dextran could be detected in the basal compartment for Caco-2 cells cultured alone (first column to the left) or with uninfected lymphocytes (third column to the left) or HTLV-1–infected lymphocytes (fourth column to the left). Opening of the barrier (positive control) was performed by adding EDTA in the apical medium, leading to a significant increase of permeability to FITC dextran (second column to the left).
Enterocytic cell lines are not susceptible to HTLV-1 infection
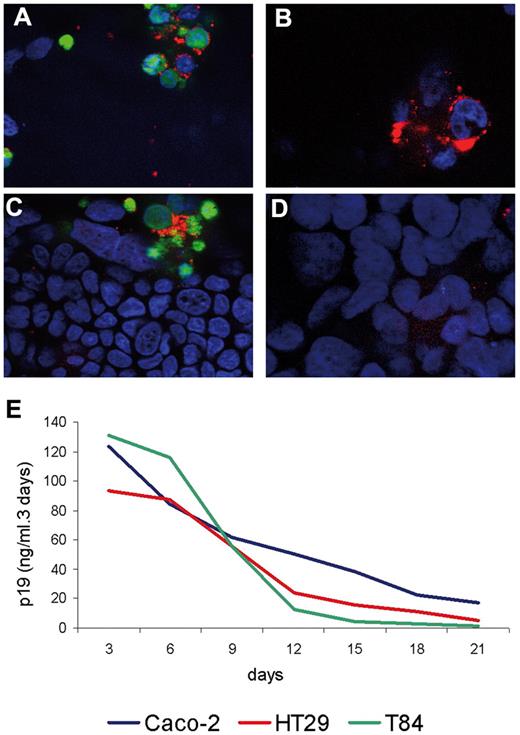
Because it could be an important pathway for the virus to cross the epithelial barrier, we assessed the susceptibility of epithelial cells to infection. During immunofluorescence studies pertaining to the viral p24 protein, no immunoreactivity was found in Caco-2 cells up to 21 days after contact with irradiated infected lymphocytes (Figure 3A-D). Equivalent results were obtained with 2 other human epithelial cell lines, HT29 and T84 (data not shown). In the 3 different cell lines tested, HTLV-1 p24 immunoreactivity was clearly restricted to the infected lymphocytes, which were identified by prior labeling with a vital dye, and HTLV-1 p24 was not present in the cytosol of the epithelial cells. Furthermore, we could not detect the formation of syncytia between epithelial cells and infected T-lymphocytes, another primary marker of HTLV-1 infection. In addition, the amount of viral p19 protein in the culture medium decreased with time for all 3 cocultured cell lines, indicating the absence of viral production by enterocytes (Figure 3E).
Assessment of susceptibility of epithelial cells to HTLV-1 infection. (A-D) Immunofluorescence observations of a coculture between Caco-2 cells and HTLV-1–infected lymphocytes (confocal microscopy at apical and basal levels of the cells). Nuclei are stained with DAPI (blue) and the p24 capsid was detected by immunofluorescence (red). In panels A and B, lymphocytes were stained (day 0) in green with the cell tracker CMFDA. Original magnification, 250×. Observation was with a Zeiss AxioVision fluorescence microscope equipped with an Apotome device. Image acquisition was with a Zeiss Axiocam camera. (A) Top view on the infected lymphocytes (apical level) of the coculture 3 days after contact. (C) Basal view on the Caco-2 cell level at day 3 after contact. (B) Top view on the level of the infected lymphocytes on day 7 after coculture. (D) Basal view of the culture showing the absence of immunoreactivity within Caco-2 cells on day 7 after coculture. (E) Production of p19 up to 21 days after coculture with irradiated HTLV-1–infected lymphocytes monitored by ELISA assay. Three different human enterocytic cell lines were tested for viral p19 production: Caco-2 (blue line), HT29 (red line), and T84 (green line) cells. For the 3 cell lines, viral protein p19 production was shown to decrease in a constant manner up to day 21.
Assessment of susceptibility of epithelial cells to HTLV-1 infection. (A-D) Immunofluorescence observations of a coculture between Caco-2 cells and HTLV-1–infected lymphocytes (confocal microscopy at apical and basal levels of the cells). Nuclei are stained with DAPI (blue) and the p24 capsid was detected by immunofluorescence (red). In panels A and B, lymphocytes were stained (day 0) in green with the cell tracker CMFDA. Original magnification, 250×. Observation was with a Zeiss AxioVision fluorescence microscope equipped with an Apotome device. Image acquisition was with a Zeiss Axiocam camera. (A) Top view on the infected lymphocytes (apical level) of the coculture 3 days after contact. (C) Basal view on the Caco-2 cell level at day 3 after contact. (B) Top view on the level of the infected lymphocytes on day 7 after coculture. (D) Basal view of the culture showing the absence of immunoreactivity within Caco-2 cells on day 7 after coculture. (E) Production of p19 up to 21 days after coculture with irradiated HTLV-1–infected lymphocytes monitored by ELISA assay. Three different human enterocytic cell lines were tested for viral p19 production: Caco-2 (blue line), HT29 (red line), and T84 (green line) cells. For the 3 cell lines, viral protein p19 production was shown to decrease in a constant manner up to day 21.
Transit of infectious virions across the epithelial barrier
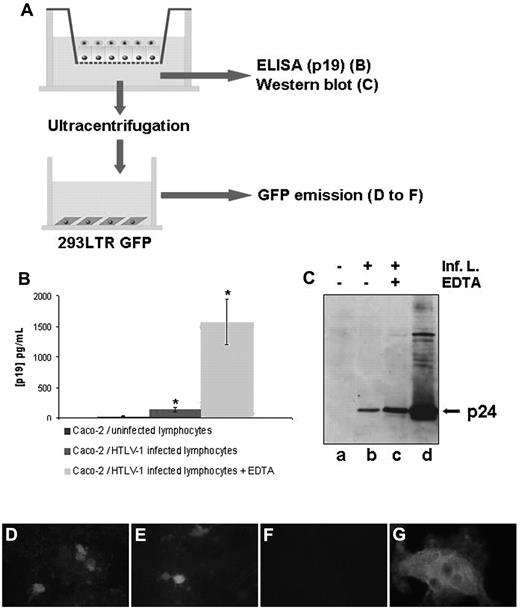
To investigate whether HTLV-1 was able to cross the epithelial barrier, we first assessed the presence of the viral p19 protein in the basal compartment of cocultures (Figure 4A). As shown in Figure 4B, ELISA carried out on the basal compartment medium revealed the presence of p19 that had been transported across the epithelial barrier in the range of 25% of the amount transported in the EDTA-treated positive control cells, in which EDTA opens the tight junctions. Interestingly, the p24 viral protein was also detected by Western blot in the basal compartment at an amount that was significant compared with the positive, EDTA-treated controls (Figure 4C).
Assessment of viral passage across a tight human epithelial barrier. (A) Experimental setting to study the viral passage after coculture. Caco-2 cells were cultured on Transwell filters before the addition of HTLV-1–infected or uninfected lymphocytes to the apical compartment. After 48 hours of coculture, basal medium was harvested for p19 measurement by ELISA (B), p24 measurement by Western blot (C), or ultracentrifuged. After resuspension, the pellet was analyzed for the presence of infectious virions by incubation with 293-LTR-GFP cells by GFP emission assay (D-F) or p24 immunoreactivity (G) of these cells. (B) The p19 viral protein content in the basal compartment was determined by an ELISA assay after a 48-hour coculture of Caco-2 cells with HTLV-1–infected lymphocytes on Transwell filters (middle column). A significant amount of p19 protein was detected compared with epithelial cells cocultured with uninfected lymphocytes (left column). *P < .05. Right column shows the positive controls, which were treated with EDTA. Mean values from 3 experiments are shown. (C) Western blot detecting p24 protein in the basal compartment after ultracentrifugation (lane a, coculture with uninfected lymphocytes; lane b, coculture with epithelial cells/HTLV-1–infected lymphocytes; lane c, coculture with HTLV-1–infected lymphocytes treated with EDTA). The whole-cell lysate of infected lymphocytes is shown in lane d. (D-G) Presence of infectious virions in the basolateral chamber. Medium of the basal compartment of a coculture (Caco-2/HTLV-1–infected lymphocytes for 48 hours) was ultracentrifuged and incubated for 5 days on 293T-LTR-GFP cells after assessment of GFP gene expression to detect transactivation by the viral Tax protein. (D) Transactivation of the viral promoter LTR by infectious virions contained in the basal compartment after coculture with HTLV-1–infected lymphocytes. € EDTA-treated positive control. (F) Negative control with the medium derived from a coculture with CEM-uninfected lymphocytes. (G) Detection of p24 immunoreactivity and syncytium formation in 293-LTR-GFP cells incubated with ultracentrifuged pellet from the basal compartment of Caco-2 coculture with HTLV-1–infected lymphocytes, as in panel D. For panels D-F, original magnification was 200×; for panel G, 400×. Observation was with a Nikon Microphot FxA (D-F) or a Leica DMRB (E) microscope equipped for fluorescence.
Assessment of viral passage across a tight human epithelial barrier. (A) Experimental setting to study the viral passage after coculture. Caco-2 cells were cultured on Transwell filters before the addition of HTLV-1–infected or uninfected lymphocytes to the apical compartment. After 48 hours of coculture, basal medium was harvested for p19 measurement by ELISA (B), p24 measurement by Western blot (C), or ultracentrifuged. After resuspension, the pellet was analyzed for the presence of infectious virions by incubation with 293-LTR-GFP cells by GFP emission assay (D-F) or p24 immunoreactivity (G) of these cells. (B) The p19 viral protein content in the basal compartment was determined by an ELISA assay after a 48-hour coculture of Caco-2 cells with HTLV-1–infected lymphocytes on Transwell filters (middle column). A significant amount of p19 protein was detected compared with epithelial cells cocultured with uninfected lymphocytes (left column). *P < .05. Right column shows the positive controls, which were treated with EDTA. Mean values from 3 experiments are shown. (C) Western blot detecting p24 protein in the basal compartment after ultracentrifugation (lane a, coculture with uninfected lymphocytes; lane b, coculture with epithelial cells/HTLV-1–infected lymphocytes; lane c, coculture with HTLV-1–infected lymphocytes treated with EDTA). The whole-cell lysate of infected lymphocytes is shown in lane d. (D-G) Presence of infectious virions in the basolateral chamber. Medium of the basal compartment of a coculture (Caco-2/HTLV-1–infected lymphocytes for 48 hours) was ultracentrifuged and incubated for 5 days on 293T-LTR-GFP cells after assessment of GFP gene expression to detect transactivation by the viral Tax protein. (D) Transactivation of the viral promoter LTR by infectious virions contained in the basal compartment after coculture with HTLV-1–infected lymphocytes. € EDTA-treated positive control. (F) Negative control with the medium derived from a coculture with CEM-uninfected lymphocytes. (G) Detection of p24 immunoreactivity and syncytium formation in 293-LTR-GFP cells incubated with ultracentrifuged pellet from the basal compartment of Caco-2 coculture with HTLV-1–infected lymphocytes, as in panel D. For panels D-F, original magnification was 200×; for panel G, 400×. Observation was with a Nikon Microphot FxA (D-F) or a Leica DMRB (E) microscope equipped for fluorescence.
To determine whether these viral proteins could reflect the passage of infectious virions across the epithelium, basolateral culture supernatants were collected 48 hours after coculture and ultracentrifuged. The pellets were resuspended and added to 293-LTR-GFP reporter cells. These cells are stably transfected with a plasmid that encompasses the GFP reporter gene under the control of the HTLV-1 LTR promoter. Therefore, transactivation of the viral LTR promoter is monitored through fluorescent emission of green light. Five days after the addition of potential HTLV-1 virions to the 293-LTR-GFP cells, transactivation of the viral promoter was clearly seen (Figure 4D), with 43 positive cells in 10 randomly chosen microscopic fields and 75 positive cells in EDTA-treated samples (Figure 4E). In addition, infection of these cells could be determined by the presence of p24-immunoreactive syncytia (Figure 4G), whereas no GFP+ cells could be observed in 293-LTR-GFP cells incubated with pellets from basolateral medium of Caco-2/CEM cell coculture (Figure 4F); syncytia and p24 immunoreactivity were also not observed (data not shown). This experiment provides evidence for the presence of infectious virions in the basolateral chamber of the Transwell filters after coculture.
HTLV-1 virions cross the tight epithelial barrier by transcytosis
The appearance of infectious HTLV-1 virions in the basolateral culture medium could be the result of transcytotic passage of the virus across the epithelial monolayer. We first assessed the effect of temperature on HTLV-1 transport across the epithelial barrier because transcytosis is an active process that is inhibited at 4°C. For this purpose, cocultures were incubated for 2 hours at either 37°C or 4°C. Using ELISA, p19 could be readily detected in the basal compartment as early as 2 hours after coculture at 37°C. At 4°C, the amount of p19 transported to the basal compartment was reduced by 85% compared with at 37° (Figure 5B), consistent with the criteria that define transcytosis.
Transcytosis of HTLV-1 virions across the epithelial cell monolayer. (A) Experimental setting to study the viral passage after coculture. Caco-2 cells were cultured on Transwell filters before the addition of HTLV-1–infected or uninfected lymphocytes (48-hour cultures) to the apical compartment. (B) Temperature dependence of the viral p19 transport across the monolayer. Cocultures of differentiated Caco-2 cells with HTLV-1–infected lymphocytes were incubated at either 4°C or 37°C. Determination of the amount of p19 protein in the basal compartment of the Transwell devices after 2 hours of contact with lymphocytes was by ELISA assay. Left column, coculture of Caco-2 cells/uninfected lymphocytes; middle column, coculture of Caco-2 cells/infected lymphocytes pretreated with EDTA (positive control); right column, coculture of Caco-2 cells/infected lymphocytes. Results are from 3 different experiments performed at 37°C (left) or 4°C (right). *P < .05. (C-E) Immunogold staining for HTLV-1 p24 protein in the cytoplasm of Caco-2 cells cocultured with infected lymphocytes by transmission electron microscopy. After 2 hours of contact between Caco-2 cells and HTLV-1–infected lymphocytes, cell monolayers on Transwell filters were fixed by ultrarapid cooling, sectioned, and immunogold labeled for the p24 capsid protein. (C-D) Immunoreactivity of HTLV-1 p24 Gag protein in specific clusters of gold particles (black arrow in C) in the cytoplasm of Caco-2 cells cocultured with infected lymphocytes. (E) No such clusters of gold particles were detected in the cytoplasm of Caco-2 cells cocultured with uninfected lymphocytes. Scale bar indicates 500 nm in panels C and E and 50 nm in panel D. Observation was with a JEOL 1010 transmission electron microscope operating at 80 kV. Image acquisition was with a Nikon CCD camera.
Transcytosis of HTLV-1 virions across the epithelial cell monolayer. (A) Experimental setting to study the viral passage after coculture. Caco-2 cells were cultured on Transwell filters before the addition of HTLV-1–infected or uninfected lymphocytes (48-hour cultures) to the apical compartment. (B) Temperature dependence of the viral p19 transport across the monolayer. Cocultures of differentiated Caco-2 cells with HTLV-1–infected lymphocytes were incubated at either 4°C or 37°C. Determination of the amount of p19 protein in the basal compartment of the Transwell devices after 2 hours of contact with lymphocytes was by ELISA assay. Left column, coculture of Caco-2 cells/uninfected lymphocytes; middle column, coculture of Caco-2 cells/infected lymphocytes pretreated with EDTA (positive control); right column, coculture of Caco-2 cells/infected lymphocytes. Results are from 3 different experiments performed at 37°C (left) or 4°C (right). *P < .05. (C-E) Immunogold staining for HTLV-1 p24 protein in the cytoplasm of Caco-2 cells cocultured with infected lymphocytes by transmission electron microscopy. After 2 hours of contact between Caco-2 cells and HTLV-1–infected lymphocytes, cell monolayers on Transwell filters were fixed by ultrarapid cooling, sectioned, and immunogold labeled for the p24 capsid protein. (C-D) Immunoreactivity of HTLV-1 p24 Gag protein in specific clusters of gold particles (black arrow in C) in the cytoplasm of Caco-2 cells cocultured with infected lymphocytes. (E) No such clusters of gold particles were detected in the cytoplasm of Caco-2 cells cocultured with uninfected lymphocytes. Scale bar indicates 500 nm in panels C and E and 50 nm in panel D. Observation was with a JEOL 1010 transmission electron microscope operating at 80 kV. Image acquisition was with a Nikon CCD camera.
To confirm the transcytotic passage of HTLV-1, we performed electron microscopy ultrastructural and immunogold staining analyses. Cocultures were instigated by incubation of epithelial cells for 2 hours with HTLV-1–infected lymphocytes or control uninfected cells, and were subsequently fixed rapidly at a very low temperature to preserve antigen-binding sites. The specimens were then subjected to p24 capsid protein immunogold labeling and observed by transmission electron microscopy. p24-immunoreactive spherical structures were detected in the cytoplasm of Caco-2 cells cocultured with infected lymphocytes (Figure 5C-D). Although membrane structures were not preserved by the cryofixation technique used, the size of such immunogold-labeled structures was found to be in the average range of HTLV-1 virions, > 80 nm. Gold particles in such “clusters” were never visualized in controls (cocultured with uninfected lymphocytes; Figure 5E). When enterocytes were incubated with cell-free viral particles, labeling could never be visualized, suggesting that the cell-to-cell contact between T lymphocytes and epithelial cells was necessary. These observations confirm the transcellular nature of the HTLV-1 passage across the tight epithelium.
DCs as potential targets after HTLV-1 epithelial transcytosis
Because DCs are found within Peyer patches and the lamina propria in close contact to the epithelial cell barrier in the digestive tract mucosa, we investigated their potential role by incorporating them into the experimental setting. Caco-2 cells were grown as usual on Transwell filters until differentiation, turned upside down, and DCs were subsequently incubated on the inversed filter, thus facing the basolateral side of the epithelial cells (Figure 6A). After 4 hours, filters were turned again and infected lymphocytes were added to the apical pole. TER was not affected after the addition of the DCs (data not shown).
HTLV-1 transported across the tight epithelial monolayer is able to infect productively human DCs. (A) Scheme depicting the experimental procedure. Briefly, Caco-2 cells were grown as usual on Transwell filters until differentiation, at which time filters were turned upside down and DCs were seeded on the inversed filter. After 4 hours, filters were turned again and HTLV-1–infected lymphocytes were added as usual to the apical pole to initiate triculture. TER was checked regularly to confirm the tightness of the cellular barrier. (B,D) Western blot and FACS analysis of the presence of the viral p24 protein within DCs. After 4 days of coculture, DCs were harvested by detaching from the filter by centrifugation (200g) and examined for the p24 viral protein. (B) Western blot assay showing a signal for the p24 protein in DCs (lane c) compared with the whole HTLV-1–infected lymphocytes cell lysate (lane a) or DCs cocultured with Caco-2/uninfected lymphocytes (lane d) and the positive control treated with EDTA (lane b). (D) Flow cytometric analysis revealed 40% DCs positive for the p24 capsid protein (green curve) after coculture underneath the Transwell with Caco-2 and HTLV-1–infected lymphocytes. (C) Localization of HTLV-1 p24 protein within DCs by immunofluorescence. DCs were labeled in green with Cell Tracker vital dye before coculture. After 3 days of contact, immunofluorescence directed against the p24 protein was performed (red). Shown is the lower side of the filter. Immunoreactivity (red) was detected either in the dendrites (left) or in the cell body (right) of DCs. Original magnification was 250×. Observation was with a Zeiss AxioVision fluorescence microscope equipped with an Apotome device. Image acquisition with a Zeiss Axiocam camera. (F) Production of viral p19 protein by DCs harvested from tricultures. Four days after contact, DCs were harvested by centrifugation (200g) from basal side of the Transwell filters, seeded in fresh medium for 4 days, and viral p19 protein was measured in the medium at days 1 and 3 after culture by ELISA assay and compared with control.
HTLV-1 transported across the tight epithelial monolayer is able to infect productively human DCs. (A) Scheme depicting the experimental procedure. Briefly, Caco-2 cells were grown as usual on Transwell filters until differentiation, at which time filters were turned upside down and DCs were seeded on the inversed filter. After 4 hours, filters were turned again and HTLV-1–infected lymphocytes were added as usual to the apical pole to initiate triculture. TER was checked regularly to confirm the tightness of the cellular barrier. (B,D) Western blot and FACS analysis of the presence of the viral p24 protein within DCs. After 4 days of coculture, DCs were harvested by detaching from the filter by centrifugation (200g) and examined for the p24 viral protein. (B) Western blot assay showing a signal for the p24 protein in DCs (lane c) compared with the whole HTLV-1–infected lymphocytes cell lysate (lane a) or DCs cocultured with Caco-2/uninfected lymphocytes (lane d) and the positive control treated with EDTA (lane b). (D) Flow cytometric analysis revealed 40% DCs positive for the p24 capsid protein (green curve) after coculture underneath the Transwell with Caco-2 and HTLV-1–infected lymphocytes. (C) Localization of HTLV-1 p24 protein within DCs by immunofluorescence. DCs were labeled in green with Cell Tracker vital dye before coculture. After 3 days of contact, immunofluorescence directed against the p24 protein was performed (red). Shown is the lower side of the filter. Immunoreactivity (red) was detected either in the dendrites (left) or in the cell body (right) of DCs. Original magnification was 250×. Observation was with a Zeiss AxioVision fluorescence microscope equipped with an Apotome device. Image acquisition with a Zeiss Axiocam camera. (F) Production of viral p19 protein by DCs harvested from tricultures. Four days after contact, DCs were harvested by centrifugation (200g) from basal side of the Transwell filters, seeded in fresh medium for 4 days, and viral p19 protein was measured in the medium at days 1 and 3 after culture by ELISA assay and compared with control.
We first assessed susceptibility of DC to HTLV-1 by immunofluorescence studies after triculture. DCs that were previously labeled with Cell Tracker CMFDA living dye were found to be immunoreactive for the viral p24 protein (Figure 6C). Section analysis by confocal microscopy allowed distinction between the 2 sides of the filters, showing a clear localization of p24 in the cytosol of DCs and their dendrites (Figure 6C). For further analysis, DCs were harvested and subjected to Western blot after 4 days of coculture (Caco-2 cells, DCs, and lymphocytes). We detected a significant signal of p24 protein by Western blot in the lysate of the DCs (Figure 6B) compared with the positive control with EDTA. Trypsin treatment of the DCs after coculture to detach membrane-adsorbed virions did not change the intensity of the band for p24 detected by Western blot, indicating that virus uptake had taken place (data not shown). Immunoreactivity for viral p24 in DCs could also be detected by flow cytometric analysis (Figure 6D).
We also investigated whether DCs were productively infected by plating them in new medium after 4 days of coculture and measuring viral p19 production by ELISA. Viral p19 was detected at increasing concentrations over time in the medium, indicating productive infection of the DCs (Figure 6E).
Discussion
HTLV-1 is the causal agent of adult T-cell leukemia/lymphoma, tropical spastic paraparesis/HTLV-1–associated myelopathy, and other inflammatory diseases. The primary cell targets of HTLV-1 are CD4+ cells, although other cell types, such as CD8+ and B cells, vascular endothelial cells, and astrocytes, may be found to be susceptible in vivo.32 Transmission of HTLV-1 through breastfeeding is thought to be the major mode of transmission in endemic countries.33 To study the passage of HTLV-1 across a tight epithelium, in the present study, we made use of an in vitro model in which we cocultured HTLV-1–infected lymphocytes on a monolayer of human intestinal epithelial cells grown on a Transwell filters. This model is known to reproducibly display several properties characteristic of differentiated epithelial cells,34 and has been used for studies on virus interactions with the digestive tract.13
To delineate an apical compartment corresponding to the tract lumen from a basolateral compartment, which corresponds to the serosal side, Caco-2 cells were grown on Transwell filter devices until differentiation and formation of tight junctions. Formation of tight junctions was checked by electron microscopy and immunofluorescence. In addition, functional integrity of the barrier was assessed by monitoring the paracellular permeability of a 4-kDa molecule across the monolayer and the TER. Interestingly, structural and functional characteristics of the epithelial barrier remained unchanged during coculture with HTLV-1–infected lymphocytes when monitored at different time points up to 72 hours after contact. These results show that HTLV-1 passage across the monolayer does not occur as a consequence of barrier breakdown.
Lymphocyte migration was likewise assessed to determine whether this could be a possible route of HTLV-1 passage across the monolayer. Uninfected lymphocytes already penetrated the barrier at very low rate (1 per 106 lymphocytes/d), and HTLV-1–infected lymphocytes were found to exhibit an even more reduced passage rate (data not shown). These results are in contrast to what had been shown in an in vitro model using endothelial cells mimicking the blood-brain barrier, in which HTLV-1–infected lymphocytes could induce barrier breakdown and, consequently, increased paracellular permeability for lymphocytes.31
Although the integrity was kept during our experiments, we still found a significant amount of HTLV-1 proteins, namely p24 and p19, in the medium of the basolateral chamber after 2 days of coculture with infected lymphocytes. These experiments were carried out on 0.4-μm pore size filter devices, excluding any cellular passage. We investigated whether these proteins corresponded to infectious HTLV-1 virions using the reporter cell line 293-LTR-GFP. The viral transactivator protein Tax was eliminated by previous ultracentrifugation, because this protein is released in the culture medium by HTLV-1–transformed lymphocytes and could drive viral gene expression alone.35 Under these conditions, we were able to detect activation of the viral promoter and infection of these target cells, indicating that infectious viruses could be rescued in the basal compartment.
Another possibility for HTLV-1 to cross the digestive tract epithelium is via infection of the epithelial cells. Such a mechanism was suggested by Zacharopoulos et al on cultured enterocytes22 ; however, in contrast to our model, coculture was performed without assessment of barrier integrity and therefore both the apical and basolateral side of the cells were exposed to the virus. Human endothelial cells were shown to be susceptible to productive HTLV-1 infection and to form large syncytia with HTLV-1 infected lymphocytes, which is significant for the blood-brain barrier.31 In our model, observations by fluorescence and confocal microscopy showed that no p24 Gag immunoreactivity nor syncytia were detected within Caco-2 cells up to 21 days after coculture.
These results were confirmed on 2 other epithelial cell lines, HT29 and T84, which were tested in the same manner. Furthermore, p19 protein production of the 3 epithelial cell lines over a 21-day period was shown to decrease in a constant manner. The fact that the concentration of p19 measured at day 21 for the Caco-2 cells remained a little higher compared with the other 2 cell lines may be explained by increased adhesion of irradiated infected lymphocytes on enterocytes, and could be explained by differential expression of ICAM-1 adhesion molecule on the 3 epithelial cell lines, with higher values reported for Caco-2 cells.36 Such absence of infection contrasts with other viruses such as poliovirus and rotavirus, which are known to infect enterocytes,37,38 and raises the possibility of HTLV-1 putative receptor expression on the apical surface of enterocytes.39 However, in the case of HIV, no infection of Caco-2 cells was observed in the present study or in a previous study of human primary enterocytes.40 Our present results show that the epithelial cell lines tested are not permissive to HTLV-1 infection, excluding this possibility as a pathway of HTLV-1 passage through the epithelium.
These findings led to the search for other possible mechanisms of viral passage. In a model that was proposed for HIV, infectious virions penetrated the mucosal barrier by transcytosis.13,14 We were also able to detect viral proteins and infectious virions release by epithelial cells at the basolateral pole by ELISA and Western blot showing activation and infection of 293-LTR-GFP cells. These results were confirmed by inhibition of the HTLV-1 passage at low temperature. In addition, ultrastructural analysis and immunogold labeling for the p24 Gag protein revealed specific clusters of gold particles in the cytoplasm of Caco-2 cells. With this unique technique, we were able to detect p24-immunoreactive particles within the cytoplasm at early steps of infection (2 hours), although membrane profiles could not be detected easily because of limitations of the technique (cryofixation). Clusters of gold particles have never been observed in negative controls or in samples incubated with cell-free virions, leading us to the conclusion that cell contact is obligatory. This is consistent with the requirement of cell-cell contact for viral transfer between lymphocytes.41,42 Temperature dependence of the HTLV-1 passage and detection of viral particles within the Caco-2 cell cytoplasm by immunogold analysis at early steps (2 hours or less) strongly suggest transcytosis as an efficient mechanism of viral passage across a tight monolayer, which may occur in the first steps of HTLV-1 transmission. The existence of an HTLV-1 transcytosis mechanism across the enterocytes opens the question of the identification of HTLV-1 receptors that could be involved at the apical pole.
In this context, the DCs, which are located in close contact to the intestinal epithelium and able to sample the inner gut lumen by their dendrites, could play a pivotal role in HTLV-1 transmission. By incorporation of human DCs into our in vitro model in the basal compartment under the enterocyte monolayer, we investigated whether these cells could transfer virus after coculture with HTLV-1–infected lymphocytes by cis or trans infection. It has been reported previously that cell-associated and cell-free virus can productively infect DCs.23,25,27 We demonstrated in the present study that viral proteins were detected within DCs 3 days after coculture, with p24 staining in the region of the dendrites and a strong signal for p24 detected on DC extracts by Western blot and flow cytometry (40% of positive cells). To determine whether this uptake of viral proteins could result in productive infection or just in an accumulation of viral proteins in endosomes after internalization, DCs were harvested from the basal side of the filters and further cultured in the absence of enterocytes and lymphocytes. Under these conditions, viral p19 production was shown to increase over time in the medium, suggesting that our previous observations were not caused by the sole uptake and accumulation of viral proteins by DCs. Such DC infection could lead to the transfer of virus to neighboring T cells. Indeed, a transfer of infection to CD4 T cells by trans infection has been demonstrated by Jones et al.27 Interestingly, reaching DCs across the intestinal barrier could be an important strategy for HTLV-1 to achieve immune suppression of the host and viral dissemination throughout the organism.24,43
Finally, epithelial cells certainly do not present the only portal entry of HTLV-1 virions during breastfeeding across the tight epithelial monolayer. Specialized M cells, for example, which are found in the follicle-associated epithelium, have been demonstrated to transcytose poliovirus, prions, and so on,18,19 and cannot be excluded in the case of HTLV-1. Intraepithelial lymphocytes that are located between epithelial cells and macrophages could also ease the uptake of the virus contained in the maternal milk. These different possibilities will certainly be the subject of future studies, because they have never been explored in regard to the retrovirus HTLV-1. The in vitro model of HTLV-1 passage through the intestinal barrier that we developed in the present study provides a valuable and relevant tool with which to address these questions in the future.
Another remaining question concerns the location where the virus contained in the maternal milk passes after arriving in the oral cavity of the infant. Lacking an appropriate animal model for HTLV-1, no study concerning viral uptake after breastfeeding has yet been done in vivo. In a simian model of infection, simian immunodeficiency virus has been shown to spread from the pluristratified squamous epithelium of the tonsils quickly to other lymphoid organs.44 Whatever the site of HTLV-1 entry through the digestive tract (eg, tonsils, gut, etc), the present study provides the first evidence for mechanisms of HTLV-1 passage through a tight epithelium. In addition, our model provides a platform with which to study the different factors that could modulate such a passage, such as lactoferrin,45 maternal Ab titer,46 and proviral load,47,48 to gain an understanding of the nature of the mother-to-child transmission of HTLV-1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank B. de Cougny and J. M. Panaud (Service Photographie, Pasteur Institute) for help in preparing the figures and Dr K. Kean and P. G. Deliege for helpful discussions and for improving the manuscript.
This work and S.M.-L. were supported by French Agence Nationnale de la Recherche. M.D. is recipient of a fellowship from the French Ministry of Education and Research. Part of the microscopy was performed at the Dynamic Imaging Platform, Imagopole, Pasteur Institute.
Authorship
Contribution: S.M.-L. and N.F.G. designed and performed the experiments, analyzed the results, and wrote the manuscript; A.M., F.G.-B., and P.J. performed the experiments; M.-C.P. and M.D. performed the experiments and analyzed the results; O.S. and A.G. analyzed the results and wrote the manuscript; S.O. analyzed the results; and P.-E.C. designed the experiments, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.M.-L. is Unité de Virologie des Aliments et de l'Eau, Agence Nationale de Sécurité Sanitaire de l'Alimentation, Maisons-Alfort, France.
Correspondence: Pierre-Emmanuel Ceccaldi, Unité Epidémiologie et Physiopathologie des Virus Oncogènes; Département de Virologie; Institut Pasteur; 25 rue du Dr Roux; 75724 Paris Cedex 15, France; e-mail: pierre-emmanuel.ceccaldi@pasteur.fr.