Abstract
Umbilical cord blood (UCB) is used for HSCT. It is known that UCB can comprise Ag-specific T cells. Here we question whether solely transmaternal cell flow may immunize UCB. Twenty-three female UCB samples were collected from healthy mothers and analyzed for minor histocompatibility Ag HY-specific responses. Forty-two of 104 tetramerpos T-cell clones, isolated from 16 of 17 UCB samples, showed male-specific lysis in vitro. Male microchimerism was present in 6 of 12 UCB samples analyzed. In conclusion, female UCB comprises HY-specific cytotoxic T cells. The immunization is presumably caused by transmaternal cell flow of male microchimerism present in the mother. The presence of immune cells in UCB that are not directed against maternal foreign Ags is remarkable and may explain the reported clinical observation of improved HSCT outcome with younger sibling donors.
Introduction
HLA-identical sibling stem cells are used for the treatment of patients suffering from various hematologic malignant and nonmalignant diseases.1,2 A recent study suggested that transplantation outcome can be improved by taking the birth order of siblings into account.3 In this study, we hypothesize that younger siblings are better donors as they might have come into contact with nonshared Ags of their older siblings through transmaternal cell flow. Although the effect of birth order could not be confirmed,4 the possibility of transmaternal cell flow has been speculated about for a long time. A consequence of the latter cell flow was recently demonstrated in a clinical study in cord blood transplantation. Namely, recipients sharing one or more HLA Ags with their cord blood donor inherited paternal Ags had reduced relapse rates compared with those who did not.5 Because scientific evidence for this phenomenon is, as yet, scarce and indirect, it challenged us to investigate the potential immunization capacity of transmaternal-derived cells. We anticipated that such immunologic studies are best performed in umbilical cord blood (UCB) because UCB is not biased by previous unknown immunization events. Moreover, fundamental studies using pregnancy as an experiment of nature of allotolerance are of prime importance for better understanding the course of allotransplantation.
Unrelated UCB is frequently used for the treatment of pediatric and adult patients.6,7 After UCB transplantation (UCBT), the incidence of GVHD is lower, that of leukemic relapse is similar, while the incidence of opportunistic infections is higher compared with BM transplantation or HSCT.8-10 It is generally believed that the differential clinical results of UCBT are because of the “naive and less Ag-experienced” status of UCB.11 Yet, in vitro analyses of UCB samples demonstrate the presence of virus and minor histocompatibility (H) Ag-specific T cells,12-14 and of various Th and cytotoxic T-cell responses in unmanipulated UCB.15,16 Moreover, it has been shown that T cells expanded from UCB can be used for donor lymphocyte infusion as their in vitro Ag-specific responses are similar to adult peripheral blood.17
During and after pregnancy, mothers can harbor fetal cells of their offspring (microchimerism).18,19 Through transmaternal cell flow, these microchimeric cells may travel from one child to the next child. We therefore hypothesize that siblings may harbor microchimeric cells from their older sibling(s) potentially leading to immune responses.
We earlier studied the bidirectional cell flow between mothers and their offspring by minor H antigenic responses and microchimerism.14,20,21 Minor H Ags are polymorphic self-proteins presented in the context of HLA class I or HLA class II molecules. Incompatibility for minor H Ags between related and/or unrelated individuals sharing the minor H Ag-presenting HLA molecule can lead to minor H Ag-specific immune responses.22,23
Here, we show for the first time the presence of minor H Ag-experienced T cells in female UCB that are not directed against the noninherited maternal Ags, but are presumably directed against Ags of the older siblings.
Methods
Inclusion of cord blood samples and family history
Female UCB was derived from full-term singleton pregnancies of healthy single and multigravidae women after informed consent was obtained. Detailed personal and obstetric history was obtained by personal interview and questionnaire. Women with a history of blood transfusion or transplantation were excluded from the study. HLA typing was performed on whole blood of the mothers and UCB samples, and on DNA from buccal swaps of the older brothers of the UCB samples. Approval for this study was obtained from the Institutional Review Board of the Leiden University Medical Center (P09.145) in accordance with the Declaration of Helsinki.
Isolation and cloning of tetramer-positive T cells
Detection and isolation of HY-specific T cells from cord blood mononuclear cells (CBMCs) were performed as previously described.14 In short, CBMCs were isolated by Ficoll-Isopaque density gradient centrifugation and depleted for various cell subsets using CD4, CD14, CD16, CD19, and glycophorin-A (GPA) MACS beads according to the manufacturer's instructions (Miltenyi Biotec). The depleted fraction was subsequently stained with validated24 PE-conjugated HLA-A2/HY (HYA2), HLA-B7/HY (HYB7), and/or HLA-B60/HY (HYB60) tetramers, allophycocyanin-conjugated CD8, and FITC-CD45RO (BD Biosciences). CD8pos tetramer-positive (tetramerpos) T cells were isolated by a FACSAria cell sorter (BD Biosciences) and collected single cell per well in 96-well plates containing irradiated female feeder cells in IMDM (Lonza) with 10% pooled human serum (HS), 1% leucoagglutinin (Sigma-Aldrich), and 25 U/mL recombinant IL-2 (Cetus). After initial stimulation, expanding T-cell clones were harvested. Tetramerpos T-cell clones were further expanded according to the same stimulation protocol.
Functional assays
T-cell clones were kept in IMDM with 10% HS and 25 U/mL IL-2 overnight before testing of cytotoxicity and proliferation according to earlier described protocols.25 T cells were tested against the male natural ligand, that is, HLA-A2 or HLA-B7 male EBV-transformed lymphoblastoid cell lines (EBV-LCLs) or against HLA-A2 or HLA-B7 female EBV-LCLs with or without 2 μg/mL of either HLA-A2/HY (FIDSYICQV) or HLA-B7/HY (SPSVDKARAEL) peptide or their X-homologue HLA-A2/HX (FIESYVCRM) or HLA-B7/HX (SPAVDKAQAEL). Supernatant was collected after 24 and 72 hours of coculturing of EBV-LCLs with T-cell clones. Herein, cytokine production was tested using the Bio-Plex Pro Human Cytokine 17-plex Panel, for the detection of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, MCP-1 (MCAF), MIP-1β, TNF-α according to the manufacturer's description (Bio-Rad Laboratories).
Detection of male microchimeric cells
Detection of male microchimerism by Y-chromosome analysis was performed in different leukocyte subsets as described earlier.21 Briefly, CD3pos T cells, CD20pos B cells, CD14pos monocytes, and CD3negCD20negCD14negCD11cpos myeloid dendritic cells (mDC) were isolated from total CBMCs or from the remaining material after CD8 enrichment by MACS. Granulocytes were obtained after CBMC isolation by lysing RBCs with NH4CL and KHCO3 containing lysis buffer. Genomic DNA was extracted by the QIAamp DNA Blood Mini Kit according to instructions of the manufacturer (QIAGEN via Westburg BV). Y-chromosomepos microchimerism was analyzed using a one-step real-time quantitative PCR (qPCR) protocol. A second PCR detecting the human hematopoietic cell kinase gene was carried out in parallel to standardize the data. Samples were scored to be microchimerism positive when male DNA could be detected at or above the threshold of 1 male in 100 000 female cells in at least 1 leukocyte subset in a minimum of 2 independently performed qPCRs.
Results
Female cord blood samples
A total of 23 UCB samples were collected from girls with or without older brothers (Table 1). The UCB samples with older brothers expressing 1 or more of the studied HY-presenting HLA class I molecules, that is, HLA-A2, HLA-B7, or HLA-B60, were used for in vitro functional analysis (UCB 1-12). UCB samples with sufficient cell numbers were used for both functional analysis and male microchimerism analysis (UCB 5, 7, 9-11). UCB samples with older brothers not expressing 1 of the studied HY-presenting HLA–class I molecules (UCB 13-16) were used for microchimerism analysis only. Female UCB samples without older brothers were collected (UCB 17-23) as intended control UCB samples.
Female UCB characteristics
| UCB no. . | HY-presenting HLA molecule UCB . | No. of older brothers . | Shared HY-presenting molecule older male sibling . | Shared HY-presenting molecule mother . | Obstetric history, mother . |
|---|---|---|---|---|---|
| 1 | A2 | 2 | (1) A2, (2) ns | A2 | — |
| 2 | A2 | 1 | ns | A2 | MC 7 wk, MC 11 wk |
| 3 | A2 | 3 | nt | nt | MC 9 wk |
| 4 | A2 | 1 | ns | ns | MC 6 wk |
| 5 | A2, B7 | 1 | A2 | ns | — |
| 6 | A2 | 1 | A2 | A2 | MC 5-7 wk, AP 8 wk 1 girl |
| 7 | A2 | 1 | A2 | ns | 1 girl |
| 8 | A2 | 1 | ns | ns | — |
| 9 | A2 | 1 | A2 | A2 | — |
| 10 | A2, B7 | 1 | B7 | A2 | — |
| 11 | A2 | 2 | (1) A2, (2) nt | A2 | MC 6 wk |
| 12 | A2, B7, B60 | 1 | A2, B60 | A2, B7 | 1 girl |
| 13 | ns | 2 | nt | ns | MC 6 wk, AP 22 wk (♂) |
| 14 | ns | 1 | nt | nt | unknown |
| 15 | ns | 1 | ns | ns | MC 10 wk |
| 16 | ns | 1 | ns | ns | — |
| 17 | A2 | 0 | na | ns | — |
| 18 | A2 | 0 | na | ns | AP 6 wk |
| 19 | A2 | 0 | na | nt | 1 girl |
| 20 | A2 | 0 | na | nt | — |
| 21 | B7 | 0 | na | nt | — |
| 22 | ns | 0 | na | nt | — |
| 23 | ns | 0 | na | nt | — |
| UCB no. . | HY-presenting HLA molecule UCB . | No. of older brothers . | Shared HY-presenting molecule older male sibling . | Shared HY-presenting molecule mother . | Obstetric history, mother . |
|---|---|---|---|---|---|
| 1 | A2 | 2 | (1) A2, (2) ns | A2 | — |
| 2 | A2 | 1 | ns | A2 | MC 7 wk, MC 11 wk |
| 3 | A2 | 3 | nt | nt | MC 9 wk |
| 4 | A2 | 1 | ns | ns | MC 6 wk |
| 5 | A2, B7 | 1 | A2 | ns | — |
| 6 | A2 | 1 | A2 | A2 | MC 5-7 wk, AP 8 wk 1 girl |
| 7 | A2 | 1 | A2 | ns | 1 girl |
| 8 | A2 | 1 | ns | ns | — |
| 9 | A2 | 1 | A2 | A2 | — |
| 10 | A2, B7 | 1 | B7 | A2 | — |
| 11 | A2 | 2 | (1) A2, (2) nt | A2 | MC 6 wk |
| 12 | A2, B7, B60 | 1 | A2, B60 | A2, B7 | 1 girl |
| 13 | ns | 2 | nt | ns | MC 6 wk, AP 22 wk (♂) |
| 14 | ns | 1 | nt | nt | unknown |
| 15 | ns | 1 | ns | ns | MC 10 wk |
| 16 | ns | 1 | ns | ns | — |
| 17 | A2 | 0 | na | ns | — |
| 18 | A2 | 0 | na | ns | AP 6 wk |
| 19 | A2 | 0 | na | nt | 1 girl |
| 20 | A2 | 0 | na | nt | — |
| 21 | B7 | 0 | na | nt | — |
| 22 | ns | 0 | na | nt | — |
| 23 | ns | 0 | na | nt | — |
UCB indicates umbilical cord blood; ns, nonshared HY-presenting HLA molecule; nt, not tested; na, not applicable, no elderly brother present; MC, spontaneous miscarriage; AP, elective abortion; and —, no relevant events in the obstetric history.
Detection and cloning of HY tetramerpos T cells in female UCB samples
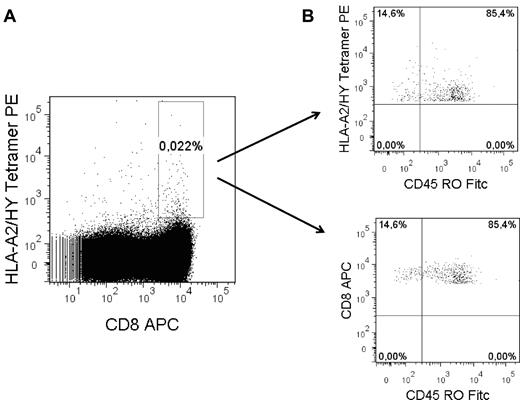
Although the precursor frequency was relatively low, tetramerpos T cells could be collected from all (n = 17) UCB samples analyzed in this study (Figure 1; Table 2). From 11 of 12 female UCB samples (92%) with at least 1 older brother, HYA2 or HYB7 tetramerpos T-cell clones were isolated. Most strikingly, HYA2 and HYB7 tetramerpos T-cell clones were isolated from 5 of 5 of our intended control female UCB samples without an older brother as well. Thus, HY tetramerpos cells could be isolated and T-cell clones could be generated from female UCB with and without older brothers.
Isolation of HY tetramerpos T cells from female UCB. (A) Example of gate setting for FACS sorting to collect all tetramerpos cells. Gate settings were based on tetramer staining intensity of a well-described HYA2 or HYB7 tetramerpos CTL clone.21 (B) Representative example of CD45RO expression of CD8posHYA2tetramerpos collected T cells.
Isolation of HY tetramerpos T cells from female UCB. (A) Example of gate setting for FACS sorting to collect all tetramerpos cells. Gate settings were based on tetramer staining intensity of a well-described HYA2 or HYB7 tetramerpos CTL clone.21 (B) Representative example of CD45RO expression of CD8posHYA2tetramerpos collected T cells.
FACS sorting and T-cell cloning results
| UCB no. . | FACS sort definitions . | No. of growing clones . | No. of HY tetramer-positive T-cell clones . | No. of clones with lysis* of male natural ligand . | No. of clones with lysis* of peptide-loaded cells . | HY microchimerism in cell subset . |
|---|---|---|---|---|---|---|
| 1 | CD8+HYA2 | 80 | 8 | 1 | 5 | nt |
| 2 | CD8+HYA2 | 24 | 3 | 1 | 1 | nt |
| 3 | CD8+HYA2 | 24 | 5 | 0 | 2 | nt |
| 4 | CD8+HYA2 | 27 | 10 | 0 | 4 | nt |
| 5 | CD8+HYA2 | 47 | 11 | 3 | 6 | Negative |
| CD8+HYB7 | 12 | 1 | 0 | 0 | ||
| 6 | CD8+HYA2 | 25 | 8 | 3 | 7 | nt |
| 7 | CD8+HYA2CD45RO+ | 27 | 1 | 0 | 1 | Monocytes |
| CD8+HYA2CD45RO− | 48 | 3 | 1 | 1 | ||
| 8 | CD8+HYA2 | 8 | 0 | na | na | nt |
| 9 | CD8+HYA2CD45RO+ | 64 | 1 | 0 | 1 | mDC, B cell |
| 10 | CD8+HYA2 | 99 | 2 | 1 | 0 | B cell |
| CD8+HYB7 | 95 | 9 | 0 | 1 | ||
| 11 | CD8+HYA2 | 31 | 1 | 0 | 1 | Negative |
| CD8+HYA2CD45RO+ | 3 | 0 | na | na | ||
| 12 | CD8+HYA2HYB7HYB60 | 80 | HYA2: 2 | 0 | 2 | nt |
| HYB7: 1 | 1 | 1 | ||||
| HYB60: 0 | na | na | ||||
| 13 | nt | na | na | na | na | Negative |
| 14 | nt | na | na | na | na | mDC |
| 15 | nt | na | na | na | na | mDC |
| 16 | nt | na | na | na | na | Monocytes |
| Total, n = | 12 | 694 | 66 of 694 tested | 11 | 33 | 6 of 9 tested |
| 17 | CD8+HYA2 | 5 | 2 | 1 | 2 | nt |
| 18 | CD8+HYA2 | 25 | 5 | 1 | 1 | nt |
| 19 | CD8+HYA2 | 81 | 1 | 0 | 0 | nt |
| 20 | CD8+HYA2 | 234 | 13 | 2 | 3 | Negative |
| 21 | CD8+HYB7 | 70 | 17 | 2 | 3 | nt |
| 22 | nt | na | na | na | na | Negative |
| 23 | nt | na | na | na | na | Negative |
| Total, n = | 5 | 415 | 38 of 415 tested | 6 | 9 | 0 of 3 tested |
| UCB no. . | FACS sort definitions . | No. of growing clones . | No. of HY tetramer-positive T-cell clones . | No. of clones with lysis* of male natural ligand . | No. of clones with lysis* of peptide-loaded cells . | HY microchimerism in cell subset . |
|---|---|---|---|---|---|---|
| 1 | CD8+HYA2 | 80 | 8 | 1 | 5 | nt |
| 2 | CD8+HYA2 | 24 | 3 | 1 | 1 | nt |
| 3 | CD8+HYA2 | 24 | 5 | 0 | 2 | nt |
| 4 | CD8+HYA2 | 27 | 10 | 0 | 4 | nt |
| 5 | CD8+HYA2 | 47 | 11 | 3 | 6 | Negative |
| CD8+HYB7 | 12 | 1 | 0 | 0 | ||
| 6 | CD8+HYA2 | 25 | 8 | 3 | 7 | nt |
| 7 | CD8+HYA2CD45RO+ | 27 | 1 | 0 | 1 | Monocytes |
| CD8+HYA2CD45RO− | 48 | 3 | 1 | 1 | ||
| 8 | CD8+HYA2 | 8 | 0 | na | na | nt |
| 9 | CD8+HYA2CD45RO+ | 64 | 1 | 0 | 1 | mDC, B cell |
| 10 | CD8+HYA2 | 99 | 2 | 1 | 0 | B cell |
| CD8+HYB7 | 95 | 9 | 0 | 1 | ||
| 11 | CD8+HYA2 | 31 | 1 | 0 | 1 | Negative |
| CD8+HYA2CD45RO+ | 3 | 0 | na | na | ||
| 12 | CD8+HYA2HYB7HYB60 | 80 | HYA2: 2 | 0 | 2 | nt |
| HYB7: 1 | 1 | 1 | ||||
| HYB60: 0 | na | na | ||||
| 13 | nt | na | na | na | na | Negative |
| 14 | nt | na | na | na | na | mDC |
| 15 | nt | na | na | na | na | mDC |
| 16 | nt | na | na | na | na | Monocytes |
| Total, n = | 12 | 694 | 66 of 694 tested | 11 | 33 | 6 of 9 tested |
| 17 | CD8+HYA2 | 5 | 2 | 1 | 2 | nt |
| 18 | CD8+HYA2 | 25 | 5 | 1 | 1 | nt |
| 19 | CD8+HYA2 | 81 | 1 | 0 | 0 | nt |
| 20 | CD8+HYA2 | 234 | 13 | 2 | 3 | Negative |
| 21 | CD8+HYB7 | 70 | 17 | 2 | 3 | nt |
| 22 | nt | na | na | na | na | Negative |
| 23 | nt | na | na | na | na | Negative |
| Total, n = | 5 | 415 | 38 of 415 tested | 6 | 9 | 0 of 3 tested |
nt indicates not tested; mDC, myeloid dendritic cell; and na, not applicable.
Defined as at least 25% of specific lysis.
HY tetramerpos T cells recognize male target cells
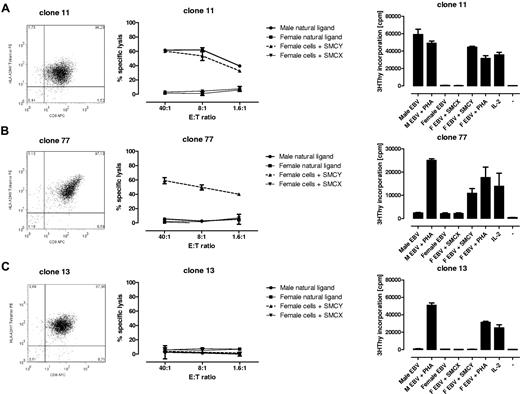
All 104 tetramerpos T-cell clones were functionally tested in vitro against the male natural ligand and against female target cells exogenously loaded with the relevant HY peptide. The results can be summarized as follows (Figure 2): 1 type of cytotoxic T-cell (CTL) clone lysed both the male natural ligand and the exogenous HY peptide-loaded female target cells (17 of 104 tetramerpos T-cell clones analyzed [16%]). The latter CTL clones also proliferated on stimulation with male cells (Figure 2A). Another type of CTL clone only lysed and proliferated on stimulation with HY peptide-pulsed targets (25 of 104 tetramerpos T-cell clones analyzed [24%]; Figure 2B). Sixty-two tetramerpos T-cell clones did not lyse, nor did they proliferate on stimulation with male target cells or HY peptide-pulsed female targets (Figure 2C). In summary, 2 types of functional HY-specific CTL clones can be isolated from female UCB recognizing either the male natural ligand and HY peptide-loaded female cells or HY peptide-loaded cells only. In addition, 62 of 104 tetramerpos T-cell clones did not show any HY Ag-specific lytic or proliferative function.
Tetramer staining and functional analyses of HY-specific T-cell clones. Three representative examples of tetramer staining by FACS, cytotoxicity, and proliferation of the T-cell clones isolated. (A) HYA2 tetramerpos T-cell clone lysed the male natural ligand (Male EBV) and HY peptide (SMCY) pulsed female EBV-LCL (F EBV) at different E:T ratios. The CTL clone proliferates on this stimulation measured by 3H thymidine incorporation. (B) Recognition of the HY peptide-pulsed female EBV-LCL only. (C) HYA2tetramerpos T-cell clone without HY-specific recognition. Note that none of the clones lysed the unloaded female EBV-LCL or HLA-A2/HX (SMCX) peptide-pulsed female EBV-LCL, but did proliferate on aspecific stimulation with 1% PHA or 25 U/mL IL-2.
Tetramer staining and functional analyses of HY-specific T-cell clones. Three representative examples of tetramer staining by FACS, cytotoxicity, and proliferation of the T-cell clones isolated. (A) HYA2 tetramerpos T-cell clone lysed the male natural ligand (Male EBV) and HY peptide (SMCY) pulsed female EBV-LCL (F EBV) at different E:T ratios. The CTL clone proliferates on this stimulation measured by 3H thymidine incorporation. (B) Recognition of the HY peptide-pulsed female EBV-LCL only. (C) HYA2tetramerpos T-cell clone without HY-specific recognition. Note that none of the clones lysed the unloaded female EBV-LCL or HLA-A2/HX (SMCX) peptide-pulsed female EBV-LCL, but did proliferate on aspecific stimulation with 1% PHA or 25 U/mL IL-2.
Phenotypic characterization of HY-specific CTL clones
Twenty-three clones were analyzed for their cytokine production using a 17-plex Luminex assay. The levels of IL-2, IL-4, IL-8, IL-13, GM-CSF, TNF-α, and IFN-γ were significantly elevated in all CTL clones analyzed on Ag-specific stimulation with either the natural ligand or HY peptide-loaded target cells (data not shown).
Next, we questioned whether CD45ROpos memory type T cells present in unmanipulated UCB before tetramer selection indeed give rise to functional HY-specific T-cell clones. CD45ROpos T cells were studied in 3 UCB samples according to the selection criteria listed in Table 2. From CD8posCD45ROposHYA2pos T cells, one high tetramerpos staining T-cell clone lysed HY peptide-loaded female target cells only (UCB 9). Another T-cell clone lysed HY peptide-loaded female target cells well and the male natural ligand at a borderline level (20% of specific lysis; UCB 7). From UCB 11, no tetramerpos T-cell clones were obtained from the CD8posCD45ROposHYA2pos fraction. As expected, the majority of all cells stained CD45ROpos during cell sorting of the CD8posHYpos T cells (Figure 1B). Likewise, on stimulation, all T cells acquired the CD45ROpos phenotype.
Male microchimeric cells are present in different cell subsets of female cord blood
Male microchimerism was determined by 1-step real-time qPCR in different cell subsets in 12 UCB smaples (Table 2). Because of the limited cell numbers in UCB, microchimerism analysis in parallel with T-cell cloning could only be performed in 6 UCB samples (UCB 5, 7, 9-11, 20). In 3 of the 5 UCB samples with older brothers from which we were able to isolate HY-specific T cells in parallel, male cells were present in 1 or more cell subset(s). In 4 other female UCB samples with older brothers (UCB 13-16), male microchimeric cells were present in 3 of 4 samples. In 1 UCB sample without older brothers (UCB 22), male microchimerism was detected at the threshold of our reliable detection limit, which we therefore scored negative. In the UCB of the other 2 firstborn girls (UCB 20 and 23), no male microchimerism was detected.
In summary, Y-chromosomepos cells can reliably be detected in female UCB samples with older brothers. Microchimeric cells of other male sources, such as the brother(s) of the mother, might be present but may be below our detection limit.
Discussion
Here we show for the first time the presence of functional HY-specific CTLs and male microchimerism in female UCB. This finding is strongly indicative for the existence of a cell flow between siblings via the mother. Most strikingly, our results extend to UCB of a firstborn girl without any older brothers. These findings further and strongly underline the immunized status of UCB, which evidently has its impact on the outcome of the clinical results of UCBT. Our current findings extend our previous results analyzing UCB wherein we demonstrated the presence of immunized T cells specific for maternal minor H Ags.14 Additional in vitro analyses show 2 types of functional Ag-specific CTLs, that is, those recognizing the natural ligand and those recognizing peptide-loaded target cells only. We earlier dissected these 2 types of functionally different Ag-specific CTLs.21,26 The latter difference could be caused by differential TCR avidity for its ligand as well as being indicative for functionally different types of T-cell clones, that is, CTL versus Treg cells. Because there is no distinct phenotype for CD8pos Tregs, a statement on the differential function of the T-cell clones we isolated currently remains pure speculation.
Alongside the potential clinical consequences of the immunized status of UCB, our results indicate that exposure of females to male Ags is probably more common than believed until now. Of note, a significant number of nulliparous females harbor male cells in their peripheral blood and tissues.27,28 In the underlying study, we detected male microchimerism in 6 (67%) of 9 female UCB samples with older brothers. In 1 female UCB sample without older brothers, male microchimerism was present at the threshold of our reliable detection limit, therefore we scored it negative. Nevertheless, it is reasonable to assume that with more sensitive detection methods male microchimerism could be detected in female cord blood without older brothers. Besides male microchimerism, functional HY-specific T cells from almost all (16 of 17) of the female UCB samples, independent of the obstetric history of the mother, could be isolated. We hypothesize that male microchimeric cells of other sources than an older brother may be capable of inducing anti-HY responses, for instance, older brothers of the mother, known or unknown miscarriages of male fetuses, or microchimerism from an unknown vanished twin brother of the UCB sample.29,30 Also paternal leukocytes or semen-derived peptides might enter the maternal and fetal circulation through uptake in the vaginal mucosa or via the decidua.31-33 Note that one of the mothers of the UCB sample without older brothers had an elective abortion after 6 weeks of pregnancy (Table 1, UCB 18), while the other mothers within this group had a blank obstetric history or only gave birth to a girl. In addition, these mothers (UCB 17, 19-23) did not have older brothers themselves.
To isolate the HY-specific T-cell clones, well-defined MHC class I/peptide complex tetramers were used.24 Tetramers have been described to activate T cells in vivo and to induce activation induced cell death in vitro.34,35 In our study, priming of HY CTLs must have taken place before tetramer selection. First, unmanipulated cells stained CD45RO positive and gave rise to functional HY-specific T cells. Second, tetramer labeling and cell sorting have been performed at 4°C thus preventing T-cell activation. Third, after tetramer selection, the T cells were stimulated in vitro in the absence of the HY Ag. Finally, previous ex vivo induction and stimulation protocols with tetramers were, at least in our hands, not successful in several cases using unprimed individuals.36 Importantly, the isolated T-cell clones were child derived as they stained with HLA-specific mAbs (kindly provided by A. Mulder, Department of Immunohematology and Blood Transfusion, Leiden University Medical Center, Leiden, The Netherlands) specific for the HLA allele the UCB sample did not share with her mother.37,38
We used the HY responses as proof of principle. Both HLA-A2– and HLA-B7–restricted HY-specific CTLs were isolated. HLA sharing of the UCB with the older brother and/or the mother appeared not necessary in all cases. However, the only UCB where T-cell clones could not be isolated did not share HLA-A2 with the UCB sample's older brother or with her mother (UCB 8). Note that nonsharing of the HY-presenting HLA molecule between mother and child implies sharing of this HLA allele with the father.
Notably, the immunization of female UCB is not restricted to HY-specific T cells only. HA-1H–specific T cells from 2 HA-1RR UCB of HA-1RR mothers were isolated as well. Several HA-1H tetramerpos T-cell lines and one HA-1H tetramerpos T-cell clone were isolated which specifically lysed HA-1H peptide-pulsed target cells (data not shown). This led us to the supposition that the transmaternal cell flow potentially causes a wealth of Ag-specific immunizations providing an explanation for the observed birth order effect in clinical transplantation.3
In conclusion, we show for the first time functional evidence for transmaternal cell flow resulting in Ag-specific T-cell priming and resulting in microchimerism in mother's offspring. As yet, we cannot estimate the implications for organ and stem cell transplantation. Evidently, our study aids the better interpretation of the clinical results in cord blood transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. L. P. van der Hoorn and A. van Lochem for UCB collection; A. Goekoop for the collection of blood samples and buccal swabs; the HLA tissue-typing laboratory of the Department of Immunohematology and Blood Transfusion for performing HLA and minor H Ag genotyping; A. Mulder for providing HLA-specific Abs; A. M. Joosten, E. van Beelen, and C. van der Keur for excellent technical assistance; M. A. W. G. van der Hoorn, G. M. de Roo, and P. P. G. van der Holst for excellent technical assistance in the FACS-sorting experiments; and Profs A. Brand, F. H. J. Claas, and J. J. van Rood for critical reading of the manuscript.
This work was financially supported by The Netherlands Organization for Scientific Research (NWO), the Macropa Foundation, and the Jan Dekker and Dr Ludgardine Bouwman Foundation.
Authorship
Contribution: M.P.D., S.A.S., and E.G. designed the research; M.P.D., E.C.B., J.P., and E.S. performed experiments and collected data; M.P.D. and E.G. analyzed results and prepared figures; and M.P.D., S.A.S., and E.G. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Miranda P. Dierselhuis, Department of Pediatrics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: m.p.dierselhuis@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal