Abstract
Protein S is a vitamin K–dependent glycoprotein, which, besides its anticoagulant function, acts as an agonist for the tyrosine kinase receptors Tyro3, Axl, and Mer. The endothelium expresses Tyro3, Axl, and Mer and produces protein S. The interaction of protein S with endothelial cells and particularly its effects on angiogenesis have not yet been analyzed. Here we show that human protein S, at circulating concentrations, inhibited vascular endothelial growth factor (VEGF) receptor 2–dependent vascularization of Matrigel plugs in vivo and the capacity of endothelial cells to form capillary-like networks in vitro as well as VEGF-A–induced endothelial migration and proliferation. Furthermore, protein S inhibited VEGF-A–induced endothelial VEGFR2 phosphorylation and activation of mitogen-activated kinase-Erk1/2 and Akt. Protein S activated the tyrosine phosphatase SHP2, and the SHP2 inhibitor NSC 87877 reversed the observed inhibition of VEGF-A–induced endothelial proliferation. Using siRNA directed against Tyro3, Axl, and Mer, we demonstrate that protein S-mediated SHP2 activation and inhibition of VEGF-A–stimulated proliferation were mediated by Mer. Our report provides the first evidence for the existence of a protein S/Mer/SHP2 axis, which inhibits VEGFR2 signaling, regulates endothelial function, and points to a role for protein S as an endogenous angiogenesis inhibitor.
Introduction
Protein S (ProS), encoded by PROS1 gene, is a vitamin K–dependent plasma glycoprotein1 that acts as an anticoagulant both as a cofactor for the anticoagulant factor-activated protein C2 (aPC) and independently of aPC.3 The importance of the anticoagulant activity of ProS is revealed by the lethal thrombotic complications suffered by homozygous PROS1-deficient mice.4 Less severe ProS deficiencies, resulting from heterozygous mutations or polymorphisms, are associated with risks for venous and arterial thrombosis, vascular calcification, and other thrombotic complications.5 Analysis of either mouse embryos from PROS1 gene mutants or from mice in which PROS1 gene was conditionally deleted in vascular smooth muscle cells revealed defects in vessel development and function not seen in mice lacking protein C (PC), implying thereby the existence of PC-independent major regulatory functions of ProS in vascular development and homeostasis.4
ProS circulates in human plasma at a concentration of ∼ 25 μg/mL in free form (40%) and in complex (60%) with C4b-binding protein (C4BP).1 In humans, most of plasma ProS is thought to be synthesized in the liver by hepatocytes.6 However, ProS is also produced at extrahepatic sites by several cell types, including macrophages,7 vascular endothelial cells (ECs),8 and vascular smooth muscle cells.9 In these cells, ProS plays no apparent role in blood coagulation, but together with its structural homolog protein Gas6, acts as an agonist for the TAM (Tyro3, Axl, and Mer) family of receptor tyrosine kinases.7,10,11 ProS has been shown to act as a ligand for Mer in the retina12 and macrophages,13 for Tyro3 in brain endothelial cells14 and neurons15 and for both Mer and Tyro3 in retinal epithelium.16 As a TAM receptor agonist, ProS regulates a variety of cellular activities, including the phagocytic clearance of apoptotic cells17 and the homeostatic regulation of the immune system.7,11
ProS/TAM interactions may play a role in vascular function, because ProS and TAM receptors are expressed by ECs,14,18 pericytes,19 and vascular smooth muscle cells.20 Recently, using tissue-specific conditional PROS1 knockout mice, it was demonstrated that extrahepatic ProS represents ∼ 45% of normal circulating ProS and that much of this residual ProS is produced by ECs.4 ECs play key roles in many processes, among which angiogenesis is a major one.21,22 Sprouting angiogenesis follows the remodeling and maturation of the primitive vascular plexus during development and plays a major role in adult life as part of both physiologic and pathologic processes.21,22 Under physiologic conditions, angiogenesis is regulated by a fine-tuning balance between endogenous promoters and inhibitors.21 The perturbation of this balance may lead to the excessive formation of aberrant and immature capillaries contributing to the pathogenesis of tumor growth and metastasis among other disorders.22
Vascular endothelial growth factor A (VEGF-A) and basic fibroblast growth factor (bFGF) are majors promoters of angiogenesis.21,22 VEGF-A is considered as a rate-limiting factor for physiologic angiogenesis and plays an important role in pathologic angiogenesis.23 VEGF-A is the agonist of tyrosine kinase receptors, VEGFR-1 and VEGFR2, the latter being the major transducer of angiogenic signaling in endothelial cells in adult life.23 On this basis, VEGF-A signaling inhibitors or VEGF-A neutralizing antibodies are used for treating pathologic angiogenesis.21-23 Although the endothelium expresses TAM receptors and is a major source of ProS,4 the pathophysiologic relevance of ProS interactions with ECs and in particularly its possible effects on angiogenesis were not as yet investigated. In the present report, we combined in vivo and in vitro approaches to investigate the role of human ProS in angiogenesis. We demonstrate that human ProS inhibits VEGFR2-dependent activation of human ECs, thereby suggesting that ProS may be a natural endogenous inhibitor of angiogenesis that may be used for targeting aberrant angiogenesis-associated disorders.
Methods
Cells
Human umbilical vein endothelial cells (HUVECs, Lonza Walkersville) were cultured in endothelial growth medium-2 (EGM-2; cc-3162, Lonza Walkersville) referred to as growth factor containing medium as described previously24 and used at early passages 1-6. When indicated, HUVEC cultures were switched to EBM-2 medium (Lonza Walkersville) referred to as a growth factor-depleted medium that was supplemented with either 0.5% FCS or ITS (insulin 10 μg/mL, transferrin 6.7 ng/mL, selenium 5.5 μg/mL). Cells were kept at 37°C in a 5% CO2 humidified incubator.
Animals
C57BL/6 male mice were used in this study. All experimental procedures involving animals were carried out in accordance with the guidelines of the French Agriculture and Forestry Ministry (decree 87849), the European Communities Council Directive (2010/63/UE), and our local ethics committee.
Materials
Human ProS was from Enzyme Research Laboratories. It was purified from fresh frozen human plasma, characterized by the supplier as a single band at 69 kDa on SDS-PAGE and described as highly pure. Human ProS from a different supplier (Calbiochem) was also tested (in both in vivo Matrigel and BrdU incorporation assays) to exclude the possibility that some of the observed effects may be the result of contaminants in the preparation. Human albumin and heparin were obtained from Sigma-Aldrich, and ITS was from Invitrogen. Basement membrane matrix (Matrigel) was purchased from BD Biosciences Discovery Labware. NSC87877 SHP 1/2 inhibitor25 was from Tocris Bioscience. Recombinant human endostatin was from Biopur. Recombinant mouse bFGF was from R&D Systems. Recombinant human bFGF and recombinant VEGF-A 165 isoform, both mouse and human, were from Invitrogen.
In vivo Matrigel plug assay
This assay was performed as described previously.26 Briefly, Matrigel was supplemented with either a mixture of proangiogenic factors (recombinant mouse bFGF and recombinant mouse VEGF-A each at 400 ng/mL and heparin at 50 units/mL) or with human ProS (either from Enzyme Research Laboratories or from Calbiochem) at 25 μg/mL, with a combination of the above or with vehicle. Eight- to 10-week-old C57BL/6 mice (n = 8) were injected subcutaneously in the flank with 0.4 mL Matrigel. Mice were killed after 7 days, and Matrigel plugs were removed, photographed, then fixed overnight in 10% formalin and embedded in paraffin. Sections (4 μm) were stained with either hematoxylin and eosin or with eosin and anti-CD31 mouse antibody (BD Pharmingen). Vascular structures or CD31-positive area were photographed using a MVX10 microscope (objective: 2×/5; 22°C; medium: Mowiol; Camera: DP25-4; cellSens Dimension Version 1.4 software, Olympus). Vascular areas identified by the presence of a lumen and red blood cells or CD31-positive area were quantified using the Fiji-win32 software and expressed as percentage vascular area per field.27,28 Data were obtained from 3 independent experiments (for each experimental point: 3 Matrigel plugs, each from a distinct mouse) and from 3 random sections of each Matrigel plug were quantified and are expressed as either vascular area percentage per field or CD31-positive area percentage per field using Fiji-win32 software as described previously.27,28
In vitro morphogenesis assay
This assay was performed as described previously.29 Matrigel was supplemented with human ProS, human endostatin, or human albumin at the indicated concentrations, coated to each well of a 24-well plate, and kept at room temperature for 4 hours to allow gel formation. HUVECs (105 cells/well) were then plated onto the Matrigel in growth factor-depleted medium (EBM-2 containing 0.5% FCS). After 24 hours, they were fixed in 4% paraformaldehyde. The 3-dimensional cell organization was photographed using MVX10 microscope (objective: 1×/1; 22°C; medium: PBS; Camera: Hamamatsu ORCA-03G; cellSens Dimension Version 1.4 software Olympus). Capillary-like structures were quantified by automatic counting in duplicate using the AngioQuant Version 1.33 software.30
Cell migration assays
Boyden chambers with 5-μm pore polycarbonate filters (Costar Corning) were coated on both sides with 0.1% gelatin were used for cell migration assays. A total of 2.5 × 104 HUVECs suspended in EBM-2 containing 0.25% BSA were seeded in the upper compartment of the Boyden chamber. The lower compartment was filled with EBM-2 medium containing 0.25% BSA supplemented with either recombinant human VEGF-A (20 ng/mL), a combination of VEGF-A (20 ng/mL) and ProS (10 μg/ml), or with vehicle. After 4 hours, the upper surface of the filter was scraped with a cotton swab, and cells that migrated to the lower surface of the filters were fixed in methanol and labeled using eosin-hematoxylin stain. Cells were photographed using a Zeiss microscope (objective 20×; medium: Mowiol; Zeiss) and counted on 15 random fields using ImageJ Version 1.39o software (National Institutes of Health).
BrdU incorporation assay
BrdU incorporation assay was used to monitor the mitogenic effects of the factors under study. HUVECs were first plated in 96-well plates at a density of 6 × 103 cells/well in EGM-2 and after 24 hours were shifted to EBM-2 and exposed to the factors under study at the indicated concentrations for 24 hours. SHP2 phosphatase inhibitor NSC87877 was used at 100μM and added 3 hours before exposing cells to VEGF-A or human ProS. Anti-ProS antibody (Dako Denmark) was used at 40 μg/mL and was incubated with ProS for 1 hour at 4°C before their addition to EC cultures. BrdU incorporation rate was measured using Cell Proliferation ELISA, BrdU chemiluminescent kit (Roche Applied Science) according to the manufacturer's instructions.
Silencing through RNA interference
To inhibit Tyro3, Axl, or Mer expression, RNA interference silencing was performed using siPORT Amine transfection agent (Ambion) according to the manufacturer's instructions. All double-stranded siRNAs were purchased from Ambion. Sequences are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). HUVECs were first plated in 6-well plates at a density of 2 × 105 cells/well in EGM-2 medium and then after 24 hours were transfected with 5 or 50nM siRNA against Tyro3, Axl, or Mer or nontargeting siRNA. Depending on the assays used, controls were siPORT amine-transfected cells (vehicle) or cells that were not transfected. Cells harvested 24 and 48 hours after transfection were used to evaluate RNA and protein expression and BrdU incorporation.
Quantitative real-time PCR
Total RNA was extracted using the NucleoSpin RNA XS kit (Machery-Nagel). Reverse transcription was performed with SuperScript II (Invitrogen) from 2 μg of total RNA according to the manufacturer's instructions. Gene expression was assessed relative to GAPDH by quantitative real-time PCR with the GeneAmp 7000 Sequence Detection System and SYBR Green chemistry (Applied Biosystems). Human GAPDH, Tyro3, Axl, and Mer primer sequences are shown in supplemental Table 2. Sensitivity and specificity of each primer couple used were tested as shown in supplemental Figure 1. Amplicons were sequenced directly using the ABI PRISM Big Dye Terminator Cycle sequencing Ready Reaction kit (Applied Biosystems) and ABI PRISM 310 automatic sequencer.
Western blotting
HUVECs were plated in 12-well plates at a density of 1 × 105 cells/well in EGM-2 medium, and then after 24 hours cells were allowed to grow in EGM-2. After 4 days, cells were exposed to the factors under study at the indicated concentrations and for the specified durations. Cells were collected by scraping and then lysed with Laemmli reducing buffer. Equivalent amounts of protein for each sample were analyzed by SDS-PAGE transferred onto PVDF membranes (Millipore) and probed with the following primary antibodies: anti-Axl, anti–phospho-Erk 1/2 Thr 202/Tyr 204, anti–Erk 1 (all from Santa Cruz Biotechnology); anti–phospho-Mer, anti–mouse Mer 101-AP (all from FabGennix); anti–human Mer (Abcam); anti-actin (Sigma-Aldrich); anti–phospho-Akt Ser473, anti-Akt, anti–phospho-SHP2 Tyr542, anti-SHP2 anti–phospho-VEGFR2 Tyr1175 and Tyr996 or anti-VEGFR2 all from Cell Signaling (Cell Signaling Technology); and anti-GAPDH (HyTest). Immunodetection was performed using chemiluminescent substrate ECL Plus (Amersham) and LAS-3000 imaging system (Fujifilm). Intensity of bands was quantified using Scion Image Version Alpha 4.0.3.2 software (Scion Corporation). Three independent experiments were done in 12-well plates. In each experiment, 3 independent wells (triplicates) received the specified agent. In total, for each experimental point, 9 cells extracts (corresponding to 9 different wells) were analyzed.
Statistical analysis
Data obtained from at least 3 independent experiments, each in triplicate unless otherwise specified, were expressed either as mean values or percentages of control values ± SD or SEM depending on the experiments performed. When indicated, statistical significance between data groups was determined by ANOVA using Prism Version 5 software (GraphPad) and considered to be significantly different at P < .05.
Results
Human ProS inhibits angiogenesis in vivo
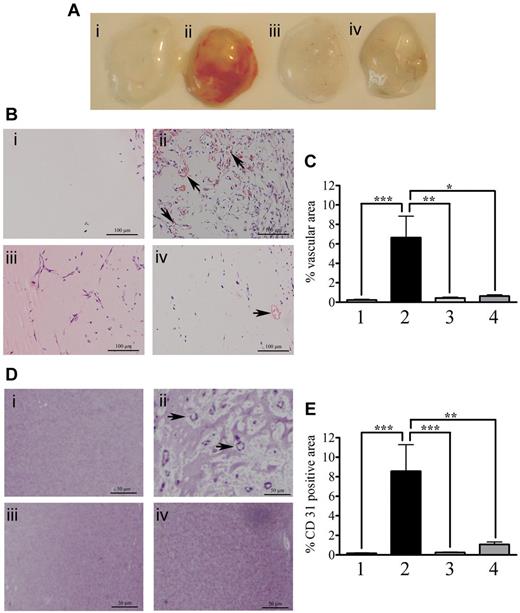
We evaluated possible effects of human ProS on angiogenesis using the in vivo Matrigel plug assay (Figure 1A). Mice were injected, subcutaneously in the flank, with either Matrigel alone (control, plug 1) or with Matrigel containing a mixture of proangiogenic factors (bFGF and VEGF-A at 400 ng/mL) in the absence (plug 2) or presence (plug 4) of 25 μg/mL human ProS or with Matrigel containing only 25 μg/mL human ProS (plug 3). After a week, Matrigel plugs were removed and analyzed. As expected, the presence of a mixture of proangiogenic factors (bFGF and VEGF-A) promoted angiogenesis (plug 2) compared with Matrigel alone (plug 1). Human ProS alone did not promote angiogenesis (plug 3), but it drastically inhibited angiogenesis induced by the mixture of proangiogenic factors (plug 4), suggesting that, under these experimental conditions, human ProS antagonized the effects of proangiogenic growth factors. Hematoxylin and eosin staining of Matrigel sections (Figure 1B) as well as quantification of vascular structures with lumens containing red blood cells (Figure 1C) confirmed a potent inhibitory effect of ProS on angiogenic factor- (bFGF and VEGF-A) induced Matrigel vascularization (Figure 1Bii compared with Figure 1Biv). Although ProS alone (Figure 1Biii) promoted cell recruitment to the Matrigel, it did not however induce the formation of vascular structures with lumens and red blood cells (Figure 1Biii) as confirmed by quantification analysis (Figure 1C) and by the absence of a red color in the Matrigel plug (Figure 1A, plug 3). Furthermore, CD31 staining (Figure 1D) and its quantification (Figure 1E) confirmed the presence of vascular structures in plugs treated with proangiogenic factors (Figure 1Dii) and their absence in plugs treated with proangiogenic factors and ProS (Figure 1Div).
Human ProS inhibits Matrigel vascularization in vivo. (A) Matrigel plugs obtained from mice that were injected subcutaneously in the flank, with 0.4 mL of Matrigel alone (lane 1, control), Matrigel containing a mixture of proangiogenic factors (400 ng/mL bFGF, 400 ng/mL VEGF-A, and 50 units/mL heparin, plug 2) or human ProS at 25 μg/mL (plug 3) or a mixture of proangiogenic factors (bFGF at 400 ng/mL, VEGF-A at 400 ng/mL, heparin at 50 units/mL) supplemented with 25 μg/mL human ProS (plug 4). (B) Representative micrographs of hematoxylin and eosin-stained sections of Matrigel plugs under the experimental conditions; Matrigel alone (i, control), Matrigel containing a mixture of proangiogenic factors (ii, 400 ng/mL bFGF, 400 ng/mL VEGF-A, and 50 units/mL heparin) or human ProS at 25 μg/mL (iii) or a mixture of proangiogenic factors (bFGF at 400 ng/mL, VEGF-A at 400 ng/mL, and heparin at 50 units/mL) supplemented with 25 μg/mL ProS (iv). The arrows indicate vascular structures with lumens and red blood cells. (C) Quantification of vascular structures with lumens and red blood cells within Matrigel sections. (D) Representative micrographs of CD31 immunstaining of Matrigel plugs under the various experimental conditions; Matrigel alone (i, control), Matrigel containing a mixture of proangiogenic factors (ii, 400 ng/mL bFGF, 400 ng/mL VEGF-A, and 50 units/mL heparin) or human ProS at 25 μg/mL (iii) or a mixture of proangiogenic factors (bFGF at 400 ng/mL, VEGF-A at 400 ng/mL, and heparin at 50 units/mL) supplemental with 25 μg/mL ProS (iv). the arrows indicate blood vessel stained with CD31. Quantifications were performed from Matrigel plugs obtained from 3 independent experiments. Within each experiment and for each experimental condition, 3 individual plugs implanted in individual animals were analyzed. From each Matrigel plug, 3 sections were stained and analyzed. Results are expressed as percentage vascular area (C) or percentage CD31-positive area (E) ± SEM, using Fiji-win32 software. ***P < .001. **P < .01. *P < .05.
Human ProS inhibits Matrigel vascularization in vivo. (A) Matrigel plugs obtained from mice that were injected subcutaneously in the flank, with 0.4 mL of Matrigel alone (lane 1, control), Matrigel containing a mixture of proangiogenic factors (400 ng/mL bFGF, 400 ng/mL VEGF-A, and 50 units/mL heparin, plug 2) or human ProS at 25 μg/mL (plug 3) or a mixture of proangiogenic factors (bFGF at 400 ng/mL, VEGF-A at 400 ng/mL, heparin at 50 units/mL) supplemented with 25 μg/mL human ProS (plug 4). (B) Representative micrographs of hematoxylin and eosin-stained sections of Matrigel plugs under the experimental conditions; Matrigel alone (i, control), Matrigel containing a mixture of proangiogenic factors (ii, 400 ng/mL bFGF, 400 ng/mL VEGF-A, and 50 units/mL heparin) or human ProS at 25 μg/mL (iii) or a mixture of proangiogenic factors (bFGF at 400 ng/mL, VEGF-A at 400 ng/mL, and heparin at 50 units/mL) supplemented with 25 μg/mL ProS (iv). The arrows indicate vascular structures with lumens and red blood cells. (C) Quantification of vascular structures with lumens and red blood cells within Matrigel sections. (D) Representative micrographs of CD31 immunstaining of Matrigel plugs under the various experimental conditions; Matrigel alone (i, control), Matrigel containing a mixture of proangiogenic factors (ii, 400 ng/mL bFGF, 400 ng/mL VEGF-A, and 50 units/mL heparin) or human ProS at 25 μg/mL (iii) or a mixture of proangiogenic factors (bFGF at 400 ng/mL, VEGF-A at 400 ng/mL, and heparin at 50 units/mL) supplemental with 25 μg/mL ProS (iv). the arrows indicate blood vessel stained with CD31. Quantifications were performed from Matrigel plugs obtained from 3 independent experiments. Within each experiment and for each experimental condition, 3 individual plugs implanted in individual animals were analyzed. From each Matrigel plug, 3 sections were stained and analyzed. Results are expressed as percentage vascular area (C) or percentage CD31-positive area (E) ± SEM, using Fiji-win32 software. ***P < .001. **P < .01. *P < .05.
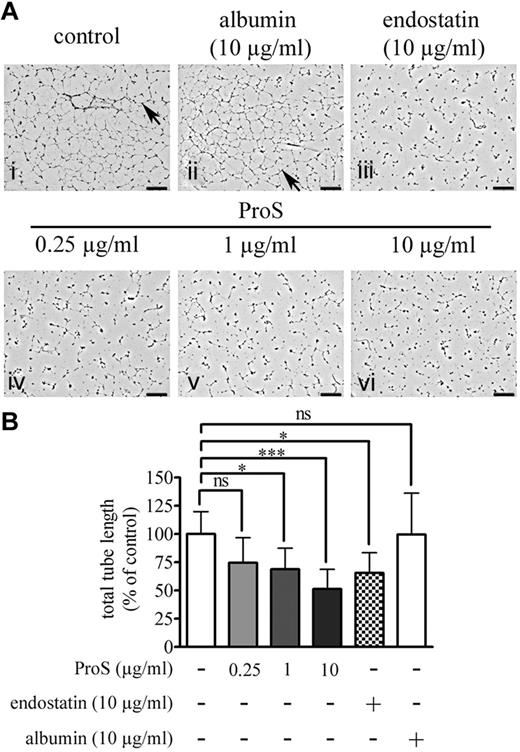
In vitro morphogenesis assays are considered as the closest to in vivo angiogenesis as they recapitulate several cellular events leading to vessel formation.29 ECs cultured on extracellular matrix preparations, such as Matrigel, spontaneously differentiate into capillary-like structures.29 To ascertain further the inhibitory effect of ProS on angiogenesis, we next tested its effects in an in vitro morphogenesis assay in which human ECs were cultured on Matrigel. Typical capillary-like structures, formed by ECs cultured on Matrigel, for 24 hours, are represented in Figure 2Ai (by arrows, control). As expected, the formation of capillary-like structures was abolished in the presence of 10 μg/mL endostatin (Figure 2Aiii), a well-known angiogenesis inhibitor.21-23 Human ProS significantly inhibited capillary-like structure formation at both 1 μg/mL (Figure 2Av, > 25% inhibition) and 10 μg/mL (Figure 2Avi, > 50% inhibition) but not at 0.25 μg/mL (Figure 2Aiv). In the presence of 10 μg/mL human ProS, ECs were unable to form interconnected tubular networks and remained mostly as single cells. At the same concentration (10 μg/mL), albumin, a serum protein with a molecular weight similar to that of ProS, had no effect on capillary-like structures (Figure 2Aii). Figure 2B provides a quantification of the in vitro morphogenesis assay in which total tube lengths were determined using AngioQuant Version 1.33 software30 and plotted as a function of the treatment. The data highlight that the inhibitory effects of 10 μg/mL human ProS are comparable with those of the well-known angiogenesis inhibitor endostatin.21-23 Supplemental Figure 2 shows that the ability of human ECs to form tubular networks was VEGF-A–dependent, as inferred by the suppression of this process by an antibody against VEGFR2. The angiogenic process in vivo (Figure 1) and the ability of ECs cultured on Matrigel to form capillary-like structures (supplemental Figure 2) were both dependent on VEGF-A and were inhibited by human ProS. Therefore, we next assessed the ability of human ProS to interfere with VEGF-A–induced VEGFR2 signaling, EC proliferation, and migration.
Human ProS inhibits the capacity of EC to form capillary-like networks in vitro. (A) The in vitro morphogenesis assay. ECs (105 cells/well) were cultured for 24 hours in EBM-2 medium containing 0.5% FCS in 24-well plates coated with Matrigel containing the indicated concentrations of human ProS, endostatin, or albumin. Cells were then fixed and the cell 3-dimensional organization was photographed using a MVX10 microscope. On the pictures, arrows indicate examples of tube formation, and each black scale represents 1 mm length. (B) The quantification of total tube length of capillary-like structures by automatic counting using the AngioQuant Version 1.33 software. Data from 4 independent experiments, each in duplicate, are expressed as percentages of control values form untreated cells ± SD. ***P < .001. *P < .05. ns indicates not significant.
Human ProS inhibits the capacity of EC to form capillary-like networks in vitro. (A) The in vitro morphogenesis assay. ECs (105 cells/well) were cultured for 24 hours in EBM-2 medium containing 0.5% FCS in 24-well plates coated with Matrigel containing the indicated concentrations of human ProS, endostatin, or albumin. Cells were then fixed and the cell 3-dimensional organization was photographed using a MVX10 microscope. On the pictures, arrows indicate examples of tube formation, and each black scale represents 1 mm length. (B) The quantification of total tube length of capillary-like structures by automatic counting using the AngioQuant Version 1.33 software. Data from 4 independent experiments, each in duplicate, are expressed as percentages of control values form untreated cells ± SD. ***P < .001. *P < .05. ns indicates not significant.
Human ProS inhibits VEGF-A–induced EC proliferation, migration, and VEGF receptor signaling.
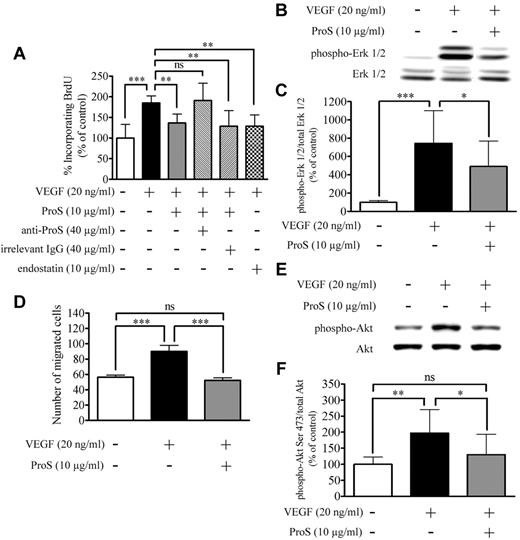
VEGF-A is a potent mitogen for ECs, and this mitogenic effect contributes to its overall angiogenic actions on ECs.21-24 Therefore, using the BrdU incorporation assay, we monitored the ability of human ProS to interfere with VEGF-A–induced proliferation in cultured ECs. Recombinant human VEGF-A at 20 ng/mL induced an increase in BrdU incorporation in ECs, which, as expected, was inhibited in the presence of 10 μg/mL of the known antiangiogenic factor endostatin21-23 (Figure 3A). Human ProS at 10 μg/mL significantly inhibited VEGF-A–induced BrdU incorporation but did not abolish it. To ascertain that the observed ProS effects are not the result of contaminants in the commercial purified ProS preparation, we tested the effects of ProS on VEGF-A–induced BrdU incorporation in the presence of an anti–human ProS antibody. As shown in Figure 3A, the anti-ProS antibody, but not an irrelevant antibody from the same species used at the same concentration (Figure 3A), reversed the inhibitory effects of ProS on VEGF-A–stimulated BrdU incorporation. In addition, we tested purified ProS obtained from a different commercial source, and this also inhibited to a similar extend VEGF-A induced BrdU incorporation (supplemental Figure 3). Moreover, commercial purified ProS, from the 2 distinct sources, consistently induced a drastic inhibition of Matrigel plug vascularization as shown in Figure 1iv. A previous report ruled out the possibility of contaminant Gas6 being present in commercial purified human ProS preparations.13 To examine further whether commercial purified human ProS used in our study contained human Gas6, we subjected this to Nano-liquid chromatography on a C18 column with a 20%-85% acetonitrile gradient coupled to mass spectrometry. The only peak observed (at 45% acetonitrile) corresponded to Human ProS mass. Finally, we used a more sensitive technique consisting of analyzing tryptic digest fragments of the protein preparations under study by nano-UPLC coupled to tandem mass spectrometry. We analyzed using this approach tryptic digest fragments obtained from commercial purified either human ProS or human Gas6. In commercial purified ProS, we detected 8 tryptic fragments that matched with human ProS sequence, and no tryptic fragments matching with human Gas6 sequence were detected (supplemental Figure 4). These results conclusively show that the observed activity of ProS could not be the result of contaminants in the purified ProS preparation.
Human ProS inhibits VEGF-A–induced EC proliferation, migration, and signaling. (A) The percentage of BrdU incorporation induced by the indicated factors. ECs were seeded at a density of 6 × 103 cells/well in 96-well plates in growth factors containing medium for 24 hours, switched to a growth factor-depleted medium containing the indicated factors at the specified concentrations. BrdU incorporation was measured by ELISA. Data obtained from 3 independent experiments each in triplicates are expressed as percentages of control ± SD. ***P < .001. **P < .01. ns indicates not significant. (B-C) Western blot analysis of MAPK-Erk1/2 activation after cultured EC exposure to the indicated factors. Subconfluent EC cultures were exposed in a growth factor–depleted medium to ProS (10 μg/mL) or vehicle for 15 minutes and then stimulated with VEGF-A (20 ng/mL) for 5 minutes. Cell cultures were then lysed, and equivalent amounts of protein form each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with either antiphosphorylated Erk 1/2 or anti–Erk 1/2 antibodies. Band intensities were quantified and are represented in panel C as a percentage of control of the ratio of phosphorylated Erk1/2 over Erk1/2 ± SD. Data were obtained from 4 independent experiments each in triplicates are expressed as percentages of control ± SD. ***P < .0001. *P < .05. (D) The number of migrated ECs induced by the indicated factors. A total of 2.5 × 104 ECs were suspended in culture medium containing 0.25% BSA, seeded in the upper compartment, and separated from the lower compartment by a 5-μm pore size polycarbonate filter coated on both sides with 0.1% gelatin. The lower compartment contained the factor under study at the indicated concentrations diluted in 0.5 mL culture medium. After 4 hours of incubation, the upper surface of the filter was scraped, and cells present in the lower compartment were fixed, stained, migrated cells were photographed under the microscope, and counted on 15 fields using ImageJ Version 1.39o software. Data were obtained from 3 independent experiments, each in triplicates, expressed as percentages of control ± SD. ***P < .0001. (E-F) Western blot analysis of Akt phosphorylation on Ser473 after cultured EC exposure to the indicated factors. Cell cultures were then lysed, and equivalent amounts of proteins from each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with either anti-Akt or anti–phosopho-Ser473 Akt antibodies. Intensity of bands was quantified and are represented in panel F as a percentage of control of the ratio of phosphorylated Akt Ser473 over Akt ± SD. Data were obtained from 3 independent experiments, each in triplicates, expressed as percentages of control ± SD. **P < .01. *P < .05. ns indicates not significant.
Human ProS inhibits VEGF-A–induced EC proliferation, migration, and signaling. (A) The percentage of BrdU incorporation induced by the indicated factors. ECs were seeded at a density of 6 × 103 cells/well in 96-well plates in growth factors containing medium for 24 hours, switched to a growth factor-depleted medium containing the indicated factors at the specified concentrations. BrdU incorporation was measured by ELISA. Data obtained from 3 independent experiments each in triplicates are expressed as percentages of control ± SD. ***P < .001. **P < .01. ns indicates not significant. (B-C) Western blot analysis of MAPK-Erk1/2 activation after cultured EC exposure to the indicated factors. Subconfluent EC cultures were exposed in a growth factor–depleted medium to ProS (10 μg/mL) or vehicle for 15 minutes and then stimulated with VEGF-A (20 ng/mL) for 5 minutes. Cell cultures were then lysed, and equivalent amounts of protein form each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with either antiphosphorylated Erk 1/2 or anti–Erk 1/2 antibodies. Band intensities were quantified and are represented in panel C as a percentage of control of the ratio of phosphorylated Erk1/2 over Erk1/2 ± SD. Data were obtained from 4 independent experiments each in triplicates are expressed as percentages of control ± SD. ***P < .0001. *P < .05. (D) The number of migrated ECs induced by the indicated factors. A total of 2.5 × 104 ECs were suspended in culture medium containing 0.25% BSA, seeded in the upper compartment, and separated from the lower compartment by a 5-μm pore size polycarbonate filter coated on both sides with 0.1% gelatin. The lower compartment contained the factor under study at the indicated concentrations diluted in 0.5 mL culture medium. After 4 hours of incubation, the upper surface of the filter was scraped, and cells present in the lower compartment were fixed, stained, migrated cells were photographed under the microscope, and counted on 15 fields using ImageJ Version 1.39o software. Data were obtained from 3 independent experiments, each in triplicates, expressed as percentages of control ± SD. ***P < .0001. (E-F) Western blot analysis of Akt phosphorylation on Ser473 after cultured EC exposure to the indicated factors. Cell cultures were then lysed, and equivalent amounts of proteins from each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with either anti-Akt or anti–phosopho-Ser473 Akt antibodies. Intensity of bands was quantified and are represented in panel F as a percentage of control of the ratio of phosphorylated Akt Ser473 over Akt ± SD. Data were obtained from 3 independent experiments, each in triplicates, expressed as percentages of control ± SD. **P < .01. *P < .05. ns indicates not significant.
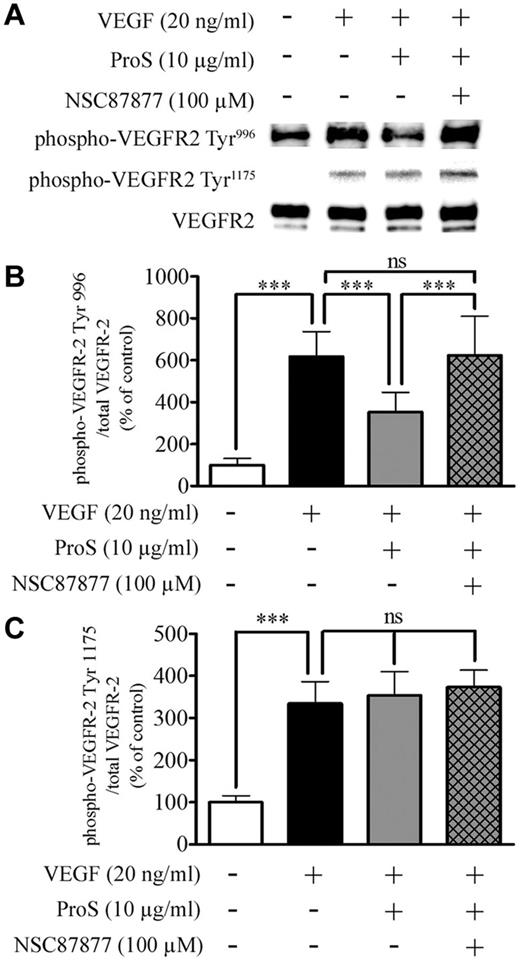
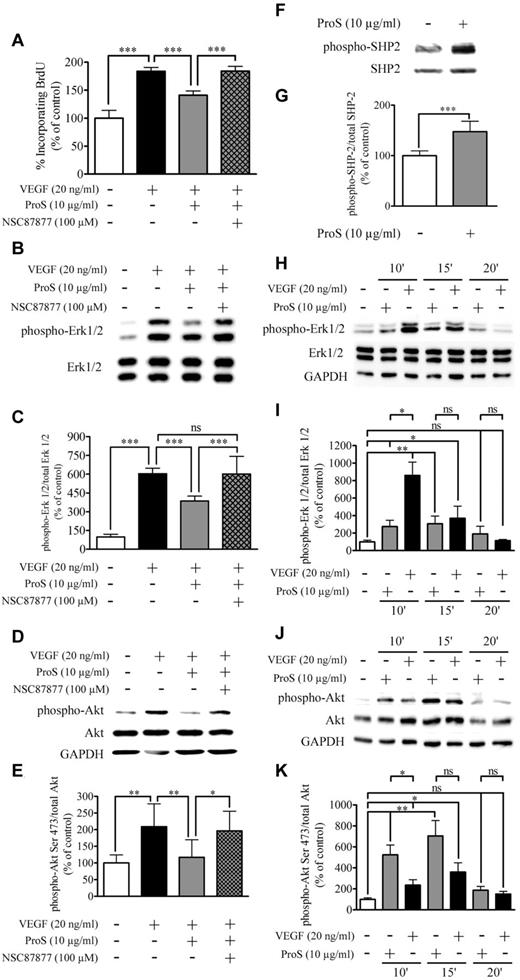
Because the activation of the MAPK-Erk1/2 signaling is an essential step in mediating the mitogenic action of VEGF-A on ECs,31 we next analyzed by Western blotting MAPK-Erk1/2 activation by VEGF-A in the absence or presence of 10 μg/mL human ProS. Figure 3B and C shows that EC exposure to human ProS for 15 minutes before VEGF-A resulted in a marked inhibition of VEGF-A–induced Erk1/2 phosphorylation/activation. The angiogenic effect of VEGF-A is in part the result of its ability to stimulate EC motility, which is an important step of vascular pattern formation.32 Therefore, we evaluated the ability of human ProS to interfere with VEGF-A–induced ECs directional migration. At 10 μg/mL, human ProS inhibited chemotaxis induced by 20 ng/mL VEGF-A by > 50% (Figure 3D). The activation of PI3K signaling and Akt phosphorylation on Ser473 is a key event associated VEGF-A–induced EC migration.31,33 Therefore, we next analyzed, by Western blotting, Akt phosphorylation on Ser473 by VEGF-A in the absence or presence of 10 μg/mL human ProS. As shown in Figure 3E and F, treatment of ECs with 10 μg/mL ProS for 15 minutes before VEGF-A resulted in a marked inhibition of VEGF-A–induced Akt phosphorylation on Ser473. To explore further the mechanisms by which human ProS antagonizes VEGF-A angiogenic signaling we analyzed, VEGFR2 phosphorylation in endothelial cells exposed to VEGF-A in the absence or presence of 10 μg/mL human ProS. Phosphorylation of Tyr996 and Tyr1175 residues, which are SHP2 sensitive and insensitive sites, respectively, was assessed.34 Figure 4 shows that ProS inhibited VEGF-A–induced VEGFR2 tyrosine phosphorylation on Tyr996 but not Tyr1175. As shown in Figure 4, the SHP2 inhibitor NSC 8787725,35 suppressed the ProS inhibitory effect on VEGFA-induced VEGFR2 phosphorylation. The SHP2 inhibitor NSC 87877 reversed the inhibitory effects of ProS on VEGF-A–induced EC proliferation (Figure 5A) and MAPK-Erk1/2 (Figure 5B-C) and Akt activation (Figure 5D-E). Figure 5F and G shows that ProS activated SHP2 phosphorylation. Therefore, SHP2 seems to play a key role in mediating ProS inhibitory effect on VEGF-A–induced signaling. We next analyzed whether ProS alone affected MAPK-Erk1/2 and Akt phosphorylation/activation. Figure 5H through K shows that ProS, on its own and independently from VEGF-A, activated both MAPK-Erk1/2 and Akt phosphorylation. Therefore, mobilization of both pathways (MAPK-Erk1/2 and Akt) by ProS alone may contribute, at least in part, to the inhibitory effect of ProS on VEGF-A-induced signaling in ECs. However, the reversal by the SHP2 inhibitor NSC 87877 of ProS inhibitory effect on VEGF-A–induced EC proliferation, VEGFR2 phosphorylation, and MAPK-Erk1/2 and Akt activation (Figure 5A-E) suggests that an SHP2-dependent mechanism also accounts for the overall inhibitory effect of ProS on VEGF-A–induced signaling, implying that the recruitment of SHP2 by the TAM receptor(s) is activated by ProS. Therefore, we next examined which TAM receptor(s) may be involved in ProS inhibitory effects on VEGF-A–induced EC activation.
Human ProS inhibits VEGF-A–induced VEGFR2 activation at SHP2 sensitive sites. (A-B) Western blotting analysis of VEGFR2 phosphorylation after cultured EC exposure to the indicated factors. Subconfluent EC cultures were preincubated in a growth factor-depleted medium with either human ProS at the indicated concentrations or with vehicle for 15 minutes and then were exposed for 5 minutes to 20 ng/mL VEGF-A. Cells were exposed to the SHP2 inhibitor NSC 87877 at 100μM for 3 hours or vehicle before ProS. Cell cultures were then lysed, and equivalent amounts of protein form each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with anti–phospho-VEGFR2 Tyr996, Tyr1175, or anti-VEGFR2 antibodies. The intensity of bands was quantified and is represented in panel C as a percentage of control of the ratio of phosphorylated VEGFR2 over VEGFR2 ± SD. Data were obtained from 3 independent experiments (each in triplicates) expressed as percentages of control ± SD. ***P < .001. ns indicates not significant.
Human ProS inhibits VEGF-A–induced VEGFR2 activation at SHP2 sensitive sites. (A-B) Western blotting analysis of VEGFR2 phosphorylation after cultured EC exposure to the indicated factors. Subconfluent EC cultures were preincubated in a growth factor-depleted medium with either human ProS at the indicated concentrations or with vehicle for 15 minutes and then were exposed for 5 minutes to 20 ng/mL VEGF-A. Cells were exposed to the SHP2 inhibitor NSC 87877 at 100μM for 3 hours or vehicle before ProS. Cell cultures were then lysed, and equivalent amounts of protein form each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with anti–phospho-VEGFR2 Tyr996, Tyr1175, or anti-VEGFR2 antibodies. The intensity of bands was quantified and is represented in panel C as a percentage of control of the ratio of phosphorylated VEGFR2 over VEGFR2 ± SD. Data were obtained from 3 independent experiments (each in triplicates) expressed as percentages of control ± SD. ***P < .001. ns indicates not significant.
Tyrosine phosphatase SHP2 is activated by ProS and is involved in the inhibitory activity of ProS on VEGF-A–mediated EC proliferation. (A) Changes in EC BrdU incorporation, in response to the indicated factors and effects of SHP2 inhibitor NSC87877 (100μM). ECs were seeded at a density of 6 × 103 cells/well in 96-well plates in growth factors containing medium for 24 hours, switched to a growth factor–depleted medium containing the indicated factors at the specified concentrations. BrdU incorporation was measured by ELISA. (B) Western blot analysis of MAPK-Erk1/2 activation state after pretreatment with NSC 87877 (100μM) for 3 hours before ProS stimulation for 15 minutes followed by VEGF-A stimulation for 5 minutes. (C) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Erk1/2 over Erk1/2. (D) Western blot analysis of Akt activation state after pretreatment with NSC 87877 (100μM) for 3 hours before ProS stimulation for 15 minutes followed by VEGF-A stimulation for 5 minutes. (E) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Akt over Akt. (F) Western blotting analysis of SHP2 phosphorylation subsequently to EC culture exposure to human ProS. Subconfluent EC cultures were exposed in a growth factor–depleted medium to 10 μg/mL human ProS or vehicle for 15 minutes. Cell cultures were then lysed, and equal amounts of proteins were analyzed by Western blotting using either an anti–phospho SHP2 or anti-SHP2 antibody. (G) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated SHP2 over SHP2. (H) Western blot analysis of MAPK-Erk1/2 activation state after treatment with ProS (10 μg/mL) for the indicated time. (I) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Erk1/2 over Erk1/2. (J) Western blot analysis of Akt activation state after treatment with ProS (10 μg/mL) for the indicated time. (K) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Akt over Akt. (A-K) Data were obtained from 3 independent experiments (3 independent cell culture) in either 96-well plates (A) or 24-well plates (B-K), each either in triplicate wells (A-G) or in duplicates (H-K), expressed as percentages of control ± SD. ***P < .001. **P < .01. *P < .05. ns indicates not significant.
Tyrosine phosphatase SHP2 is activated by ProS and is involved in the inhibitory activity of ProS on VEGF-A–mediated EC proliferation. (A) Changes in EC BrdU incorporation, in response to the indicated factors and effects of SHP2 inhibitor NSC87877 (100μM). ECs were seeded at a density of 6 × 103 cells/well in 96-well plates in growth factors containing medium for 24 hours, switched to a growth factor–depleted medium containing the indicated factors at the specified concentrations. BrdU incorporation was measured by ELISA. (B) Western blot analysis of MAPK-Erk1/2 activation state after pretreatment with NSC 87877 (100μM) for 3 hours before ProS stimulation for 15 minutes followed by VEGF-A stimulation for 5 minutes. (C) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Erk1/2 over Erk1/2. (D) Western blot analysis of Akt activation state after pretreatment with NSC 87877 (100μM) for 3 hours before ProS stimulation for 15 minutes followed by VEGF-A stimulation for 5 minutes. (E) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Akt over Akt. (F) Western blotting analysis of SHP2 phosphorylation subsequently to EC culture exposure to human ProS. Subconfluent EC cultures were exposed in a growth factor–depleted medium to 10 μg/mL human ProS or vehicle for 15 minutes. Cell cultures were then lysed, and equal amounts of proteins were analyzed by Western blotting using either an anti–phospho SHP2 or anti-SHP2 antibody. (G) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated SHP2 over SHP2. (H) Western blot analysis of MAPK-Erk1/2 activation state after treatment with ProS (10 μg/mL) for the indicated time. (I) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Erk1/2 over Erk1/2. (J) Western blot analysis of Akt activation state after treatment with ProS (10 μg/mL) for the indicated time. (K) Intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated Akt over Akt. (A-K) Data were obtained from 3 independent experiments (3 independent cell culture) in either 96-well plates (A) or 24-well plates (B-K), each either in triplicate wells (A-G) or in duplicates (H-K), expressed as percentages of control ± SD. ***P < .001. **P < .01. *P < .05. ns indicates not significant.
The tyrosine kinase receptor Mer is activated by ProS and mediates its inhibitory effect on VEGF-A–induced EC proliferation
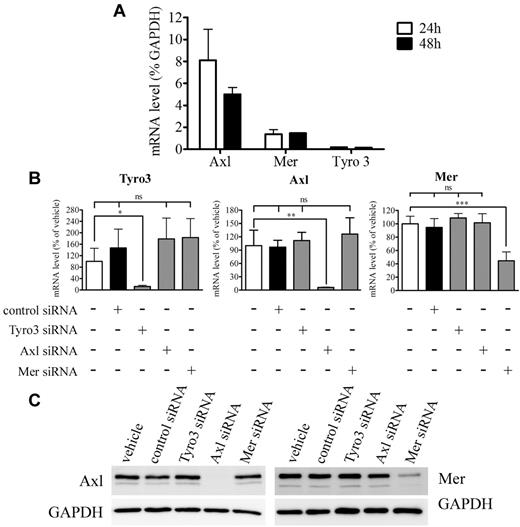
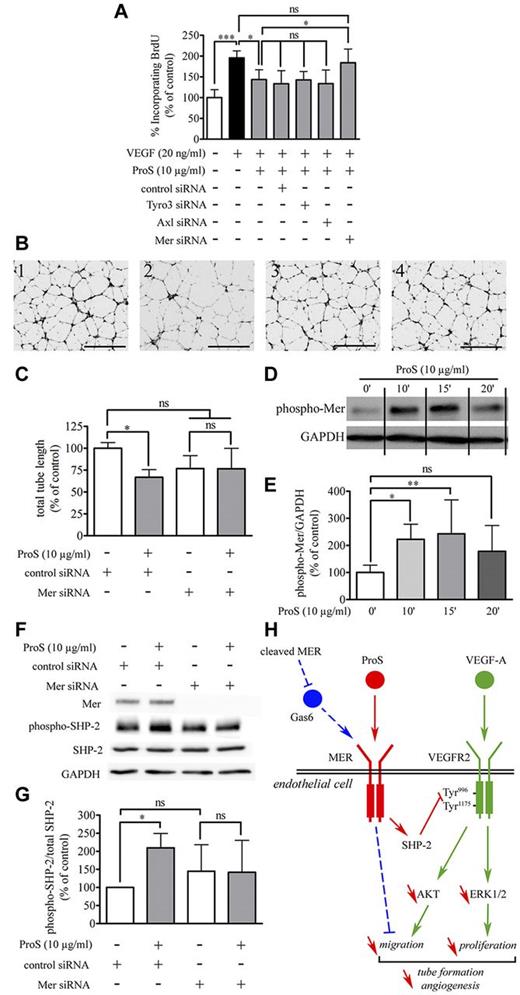
Human ProS is a potential agonist for the TAM family of receptor tyrosine kinases (Tyro3, Axl, and Mer).10 Real-time quantitative PCR analysis established that cultured EC expressed Axl, Tyro3, and Mer (Figure 6A; supplemental Table 2). In agreement with a previous report,18 Axl transcript levels were ∼ 3-fold more abundant than those for Mer and in turn, Mer transcripts were ∼ 11-fold more abundant than Tyro3 transcripts. Next, we used siRNA directed specifically against each of the 3 tyrosine kinase receptors. We show efficient and specific silencing of each transcript at both 24 and 48 hours after transfection, each of these siRNA efficiently target the transcript toward which it is specifically directed and does not interfere with the expression of 2 others (Figure 6B-C; supplemental Figure 5). Twenty-four to 48 hours are within the timing of the BrdU incorporation assay we use in our study, implying that under these conditions the ability of each of these siRNA to interfere with the inhibitory action of human ProS on VEGF-A–induced EC mitosis could be assessed. We next investigated whether any of these tyrosine kinase receptors were involved in the inhibitory effects of ProS on VEGF-A–induced EC activation. As shown in Figure 7A, silencing of either Axl or Tyro3 did not alter the inhibitory action of human ProS on VEGF-A–induced EC proliferation, whereas silencing of Mer completely suppressed it, suggesting that ProS by activating Mer tyrosine kinase receptor inhibits VEGF-A–mitogenic signaling. Furthermore, we used the in vitro morphogenesis assay described in Figure 2, which is considered as the closest to in vivo angiogenesis29 to assess the consequences of Mer silencing on the ability of human ProS to inhibit capillary-like structure formation. Figure 7B and C show that Mer silencing suppressed ProS inhibitory action on capillary-like structure formation. Supplemental Figure 6 confirms that Mer siRNA induced under these experimental conditions efficient Mer silencing within 24 and 48 hours. Accordingly, treatment of endothelial cells with human ProS induced significant Mer receptor tyrosine phosphorylation within 10 minutes, which peaked at 15 minutes and declined at 20 minutes (Figure 7D-E). Furthermore, silencing of Mer prevented the activation of SHP2 by ProS (Figure 7F-G). Because the in vivo Matrigel plug assay was performed on mice using Human ProS, we verified and confirmed, using a mouse cell line and the Mer receptor tyrosine phosphorylation assay described above, that Human ProS does activate mouse Mer tyrosine kinase receptor (supplemental Figure 7). Altogether, these data suggest that, in human ECs, human ProS activates Mer tyrosine kinase receptor, which in turn recruits/activates SHP2, which dephosphorylates VEGFR2 on Tyr996, thereby inhibiting multiple VEGF-A–dependent angiogenesis-related events as illustrated in Figure 7H.
TAM receptor expression and siRNA knockdown validation. (A) Tyro3, Axl, and Mer gene transcript levels relative to that of GAPDH as determined by quantitative real-time PCR after 24 and 48 hours of EC culture in growth factor–containing medium. (B-C) The effectiveness and the specificity of targeted siRNA in silencing Tyro3, Axl, and Mer gene expression, real-time quantitative PCR 24 hours after siRNA transfection (B) or Western blot analysis 24 hours after siRNA transfection (C). EC cultures were transfected with either the specified siRNA used at 5nM for Tyro3 and Axl or at 50nM for Mer or with vehicle (5 μL/well siPORT) or as a control with 50nM nontargeting siRNA. For panel B, cells were harvested 24 hours after transfection and total RNA extracted. Reverse transcription was performed using 2 μg of total extracted RNA. Tyro3, Axl, and Mer gene expression was assessed relatively to that of GAPDH by quantitative real-time PCR and is expressed as percentage of the same ratio in siPORT-transfected ECs. Data were obtained from 3 independent experiments each in triplicate ± SD. ***P < .001. **P < .01. *P < .05. ns indicates not significant. For panel C, equivalent amounts of protein form each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with anti-Mer, anti-Axl, or anti-GAPDH antibodies.
TAM receptor expression and siRNA knockdown validation. (A) Tyro3, Axl, and Mer gene transcript levels relative to that of GAPDH as determined by quantitative real-time PCR after 24 and 48 hours of EC culture in growth factor–containing medium. (B-C) The effectiveness and the specificity of targeted siRNA in silencing Tyro3, Axl, and Mer gene expression, real-time quantitative PCR 24 hours after siRNA transfection (B) or Western blot analysis 24 hours after siRNA transfection (C). EC cultures were transfected with either the specified siRNA used at 5nM for Tyro3 and Axl or at 50nM for Mer or with vehicle (5 μL/well siPORT) or as a control with 50nM nontargeting siRNA. For panel B, cells were harvested 24 hours after transfection and total RNA extracted. Reverse transcription was performed using 2 μg of total extracted RNA. Tyro3, Axl, and Mer gene expression was assessed relatively to that of GAPDH by quantitative real-time PCR and is expressed as percentage of the same ratio in siPORT-transfected ECs. Data were obtained from 3 independent experiments each in triplicate ± SD. ***P < .001. **P < .01. *P < .05. ns indicates not significant. For panel C, equivalent amounts of protein form each sample were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with anti-Mer, anti-Axl, or anti-GAPDH antibodies.
Mer silencing suppresses both ProS-induced SHP2 activation and inhibition of VEGF-A–induced EC BrdU incorporation and tube formation. (A) Changes in BrdU incorporation resulting from silencing of Tyro3, Axl, or Mer gene expression. ECs were seeded at a density of 6 × 103 cells/well in 96-well plates in EGM-2 for 24 hours. Cells were then transfected with either the specified siRNA used either at 5nM for Tyro3 and Axl or at 50nM for Mer or with vehicle (0.5 μL/well siPORT) or as a control with 50nM nontargeting siRNA. At 24 hours after transfection, cells were switched to EBM-2 containing the indicated factors at the specified concentrations. BrdU incorporation was measured by ELISA. Data obtained from 3 independent experiments (3 distinct independent cell culture 96-well plates) each in triplicate wells are expressed as percentages of the same ratio in siPORT-transfected ECs ± SD. ***P < .001. *P < .05. ns indicates not significant. (B) The in vitro morphogenesis assay. ECs (2 × 105 cells/well) were cultured for 24 hours in complete medium and then transfected with 5nM of either Mer or control siRNA. At 24 hours after transfection, cells were harvested and seeded at the density of 105 cells/well in EBM-2 containing 0.5% FCS and 20 ng/mL VEGF-A, in 24-well plates coated with Matrigel containing either human ProS (20 μg/mL) or vehicle. The cell 3-dimensional organization was photographed using a MVX10 microscope. Black scale bar on pictures represents 100 μm length. (C) Quantifications of total tube length of capillary-like structures by automatic counting using the AngioQuant software. Data obtained from 3 independent experiments each in duplicates are expressed as percentages of control ± SD. *P < .05. ns indicates not significant. (D) Mer receptor tyrosine phosphorylation after treatment with ProS (10 μg/mL) for the indicated time. GAPDH was used as loading control. Because the data were obtained from 3 independent experiments each in triplicate and only 1 representative result for each time point is depicted in the figure, vertical lines have been inserted to indicate repositioned gel lanes. The intensity of bands was quantified and is represented in panel E as a percentage of control of the ratio of phosphorylated Mer over GAPDH ± SD. **P < .01. *P < .05. ns indicates not significant. (F) Western blotting analysis of Mer receptor expression and changes in EC response to ProS, in term of SHP2 activation, after silencing of Mer. (G) The intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated SHP2 over SHP2; some error bars were too small to be visible. Data obtained from 4 independent experiments each in duplicate are expressed as percentages of control ± SD. *P < .05. ns indicates not significant. (H) The proposed mechanism through which ProS interferes with VEGF-A-induced VEGFR2 signaling. VEGF-A triggers VEGFR2, Akt, and Erk 1/2 activation, leading to EC migration proliferation and subsequently to vascular tube formation (in green). ProS activates its tyrosine kinase receptor Mer, leading to SHP-2 activation, which dephosphorylates VEGFR2 on the SHP2-sensitive site Tyr996, thereby inhibiting VEGFR2 recruitment of Akt and Erk 1/2 pathways and subsequently VEGFR2 induced EC migration and proliferation necessary for tube formation and angiogenesis (in red). A recent report45 has described that, similarly to ProS, its structural homolog Gas6 activates Mer tyrosine kinase receptor, leading to the inhibition of EC migration and angiogenesis (in blue). Tumor cells release soluble Mer, which acts as a decoy receptor for Gas6, thereby reducing the suppressive effects of Gas6 on endothelial cell recruitment.
Mer silencing suppresses both ProS-induced SHP2 activation and inhibition of VEGF-A–induced EC BrdU incorporation and tube formation. (A) Changes in BrdU incorporation resulting from silencing of Tyro3, Axl, or Mer gene expression. ECs were seeded at a density of 6 × 103 cells/well in 96-well plates in EGM-2 for 24 hours. Cells were then transfected with either the specified siRNA used either at 5nM for Tyro3 and Axl or at 50nM for Mer or with vehicle (0.5 μL/well siPORT) or as a control with 50nM nontargeting siRNA. At 24 hours after transfection, cells were switched to EBM-2 containing the indicated factors at the specified concentrations. BrdU incorporation was measured by ELISA. Data obtained from 3 independent experiments (3 distinct independent cell culture 96-well plates) each in triplicate wells are expressed as percentages of the same ratio in siPORT-transfected ECs ± SD. ***P < .001. *P < .05. ns indicates not significant. (B) The in vitro morphogenesis assay. ECs (2 × 105 cells/well) were cultured for 24 hours in complete medium and then transfected with 5nM of either Mer or control siRNA. At 24 hours after transfection, cells were harvested and seeded at the density of 105 cells/well in EBM-2 containing 0.5% FCS and 20 ng/mL VEGF-A, in 24-well plates coated with Matrigel containing either human ProS (20 μg/mL) or vehicle. The cell 3-dimensional organization was photographed using a MVX10 microscope. Black scale bar on pictures represents 100 μm length. (C) Quantifications of total tube length of capillary-like structures by automatic counting using the AngioQuant software. Data obtained from 3 independent experiments each in duplicates are expressed as percentages of control ± SD. *P < .05. ns indicates not significant. (D) Mer receptor tyrosine phosphorylation after treatment with ProS (10 μg/mL) for the indicated time. GAPDH was used as loading control. Because the data were obtained from 3 independent experiments each in triplicate and only 1 representative result for each time point is depicted in the figure, vertical lines have been inserted to indicate repositioned gel lanes. The intensity of bands was quantified and is represented in panel E as a percentage of control of the ratio of phosphorylated Mer over GAPDH ± SD. **P < .01. *P < .05. ns indicates not significant. (F) Western blotting analysis of Mer receptor expression and changes in EC response to ProS, in term of SHP2 activation, after silencing of Mer. (G) The intensity of bands was quantified and is represented as a percentage of control of the ratio of phosphorylated SHP2 over SHP2; some error bars were too small to be visible. Data obtained from 4 independent experiments each in duplicate are expressed as percentages of control ± SD. *P < .05. ns indicates not significant. (H) The proposed mechanism through which ProS interferes with VEGF-A-induced VEGFR2 signaling. VEGF-A triggers VEGFR2, Akt, and Erk 1/2 activation, leading to EC migration proliferation and subsequently to vascular tube formation (in green). ProS activates its tyrosine kinase receptor Mer, leading to SHP-2 activation, which dephosphorylates VEGFR2 on the SHP2-sensitive site Tyr996, thereby inhibiting VEGFR2 recruitment of Akt and Erk 1/2 pathways and subsequently VEGFR2 induced EC migration and proliferation necessary for tube formation and angiogenesis (in red). A recent report45 has described that, similarly to ProS, its structural homolog Gas6 activates Mer tyrosine kinase receptor, leading to the inhibition of EC migration and angiogenesis (in blue). Tumor cells release soluble Mer, which acts as a decoy receptor for Gas6, thereby reducing the suppressive effects of Gas6 on endothelial cell recruitment.
Discussion
ProS, by acting as an agonist for the TAM family of receptor tyrosine kinases (Tyro3, Axl, and Mer),10 regulates a variety of cellular processes, ranging from cell proliferation,9 survival,14 phagocytic clearance of apoptotic cells,17 and the homeostatic regulation of the immune system.36 The production of ProS at several extrahepatic sites, including the vasculature,7-9,37 suggests that, besides and independently of its role in blood coagulation, ProS may act as an autocrine/paracrine factor.
In the present study, we describe, for the first time to our knowledge, a human ProS inhibitory pathway, which impedes the angiogenic program activated by VEGF-A. We provide evidence that human ProS inhibits the vascularization of a Matrigel plug in vivo and the capacity of ECs to form capillary-like networks as well as VEGF-A–dependent EC migration, mitosis, and signaling (VEGFR2, MAPK-Erk1/2, and Akt phosphorylation) in vitro. Depending mainly on the cell type studied and the identity of the tyrosine kinase receptor involved, ProS exhibits several cell activities in different contexts. In the vascular system, it induces vascular smooth muscle cell proliferation9 and acts as a survival factor for brain endothelial cells.14 ProS circulates in human plasma at a concentration of ∼ 25 μg/mL, which is within the range (1-25 μg/mL) we used in all our in vitro and in vivo assays. Because substantial amounts of ProS are produced by vascular wall components8,9 as well as by some cancer cells,38,39 the antiangiogenic effect of human ProS, we report in the present study, may constitute an autocrine/paracrine inhibitory mechanism for angiogenesis contributing thereby to a tuning balance between endogenous promoters and inhibitors of angiogenesis. Similarly to ProS, angiostatin, which is a potent natural antiangiogenic factor, is produced by primary tumors.40
Using TAM receptor gene silencing, we demonstrate that the tyrosine kinase receptor Mer mediates ProS inhibitory effects on VEGF-A activity. Because cytosolic protein tyrosine phosphatases containing Src homology domain-2 are potent regulators of intracellular pathways activated by many tyrosine kinase receptors, we tested the hypothesis that SHP2 could be recruited to mediate an inhibitory effect of ProS-Mer axis on VEGF-A–induced EC mitosis. Both indirect (ProS dephosphorylates VEGR-2 on Tyr996 but not Tyr1175 residues, which are SHP2 sensitive and insensitive sites, respectively) and direct (activation of SHP2 by ProS and the suppression of this activation by Mer silencing) observations provide the first evidence, to our knowledge, that human ProS/Mer axis recruits the tyrosine phosphatase SHP2. Human ProS induced tyrosine phosphorylation of both Mer receptor and SHP2, and Mer silencing suppressed the ability of ProS to induce SHP2 phosphorylation and significantly altered its inhibitory action on VEGF-A–induced EC proliferation and capillary-like structure formation (Figure 7). It was reported that Axl stimulation by the structural homolog of ProS, Gas6, activates SHP2, thereby inhibiting VEGF-A–dependent activation of both VEGFR2 and the angiogenic program in vascular endothelial cells.29 More recently, Ruan and Kazlauskas demonstrated that VEGF-A–dependent activation of VEGFR2 and recruitment of Src family kinases engages the receptor tyrosine kinase Axl to trigger ligand-independent autophosphorylation that promotes activation of Akt.41 The evidence we provide for the existence of a ProS/Mer/SHP2 axis, which inhibits VEGF-A–mediated VEGFR2, MAPK Erk1/2, and Akt activation, suggests that, besides Axl, Mer tyrosine kinase receptors is also a key regulator of VEGF-A–dependent angiogenesis. The occurrence of ligand-independent autophosphorylation of Mer tyrosine kinase receptor that may regulate Akt, similarly to that reported for Axl,41 remains to be examined.
Uehara and Shacter demonstrated that disulfide-linked ProS oligomers stimulate phagocytosis of apoptotic cells by human macrophages by inducing the dimerization and activation of macrophage Mer tyrosine kinase receptor.42 This study also established that phosphatidylserine exposed on the outer surface of apoptotic cells provides a scaffold for intermolecular ProS interactions and its oxidative oligomerization.42 Free ProS stimulates phagocytosis of apoptotic cells, whereas ProS in complex with C4BP1 has an inhibitory effect on this process, implying that on macrophages C4BP inhibits the interaction between ProS and its receptors.43 It will be interesting in the future to investigate whether ProS oligomerization is essential for its presently described new role in inhibiting VEGF-A–induced EC activation and angiogenesis, whether phosphatidylserine exposed on the outer surface of apoptotic cells that may be present within EC cultures in vitro or within the vessel wall in vivo provides a scaffold for intermolecular ProS oxidative oligomerization and how C4BP may be affecting this newly described role of ProS on angiogenesis. Sather et al described the presence of a soluble form of Mer tyrosine kinase receptor, consisting of its extracellular domain, produced by proteolytic cleavage, and found in human plasma and cell culture media.44 While the present report was under preparation, a study by Png et al45 convincingly demonstrated that Mer tyrosine kinase receptor is the endothelial receptor that mediates the Gas6-suppressive effect on metastatic endothelial recruitment and metastatic angiogenesis, and that soluble Mer tyrosine kinase receptor from metastatic cells acts as a decoy receptor for Gas6, thereby reducing the suppressive effects of Gas6 on endothelial cell recruitment. The present report, by revealing that Mer tyrosine kinase mediates ProS inhibitory action on VEGF-A angiogenic activities, does not only confirm some of the findings of the study by Png et al45 but also extends them by identifying (1) the activation of Mer tyrosine kinase receptor by ProS as an additional mechanism for inhibiting endothelial cell recruitment and angiogenesis, and (2) SHP2 phosphatase as an intracellular mediator of ProS/Mer antiangiogenic action (Figure 7H).
ProS and Gas6 have a strong amino acid sequence similarity, a similar domain organization, share some activities, but have many differences in functions. ProS plays a critical role as an anticoagulant,1-5 whereas Gas6 has no anticoagulant function but plays an important role in platelet aggregation.46 Human Gas6 is a potent, whereas human ProS is a weak, ligand for TAM tyrosine kinase receptors.47,48 The plasma concentration of Gas6 is ∼ 20 ng/mL, meaning that it is ∼ 1000-fold lower than that of ProS.49 Therefore, differences between Gas6 and ProS with regards to their affinity for TAM RTKs47,48 may be compensated by differences between Gas6 and ProS both in term of their plasma concentration and their spatiotemporal expression, suggesting that, depending on the context, both Gas6 and ProS could activate TAM tyrosine kinase receptors and regulate specific cellular processes.
We also demonstrate in the present study that ProS inhibits VEGFA-induced VEGFR2, Erk1/2 MAPK, and Akt activation; the 2 later pathways may be primarily linked to ProS inhibitory action on VEGF-A mitogenic and chemotactic effects on ECs, respectively. Alternatively, the inhibitory action of ProS on VEGF-A induced MAPK-Erk1/2 and Akt activation may relate to the regulation by ProS of additional VEGF-A–dependent events, such as vascular permeability. In this context, the presently described inhibitory effect of ProS on angiogenesis and EC motility along with its known survival effect on ECs14 may seem contradictory. However, taking into account that migrating cells could undergo apoptosis by anoikisis,50 inhibition of EC motility by ProS unraveled in the present study may be part of integrated effect of ProS to promote EC survival and vessel wall stability. The recent finding of defects in vessel development and function in mice embryos from ProS gene mutants or from mice in which ProS gene was conditionally deleted in vascular smooth muscle reinforces the hypothesis of a crucial role of ProS in vessel wall stability.4 The observation that ProS/Mer axis inhibits macrophage scavenger receptor A–mediated AcLDL uptake and scavenger receptor A expression in human monocyte-derived macrophages,13 provided the basis for a regulatory role of ProS/Mer in atherogenesis and atherothrombosis. Therefore, besides the direct role of ProS/Mer axis on endothelial cells, revealed in the present report, ProS/Mer axis, by acting on other components of the vessel wall,13 may also play an important role for its injury and repair.
Besides its potential for being used as part of an antiangiogenic therapy in human cancers and other angiogenesis-dependent disorders, the antiangiogenic effect of ProS/Mer axis we report in the present study may also form the framework of future studies aiming at elucidating further the involvement of ProS produced within the vessel wall in vascular development, control of vessel barrier function, and vessel wall integrity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Cantereau and IMAGEUP facility of the University of Poitiers for microscopy; Laboratoire d'Anatomie Pathologique Véterinaire, Amboise for Matrigel section staining; and W. Kaaki and PROTEOMEUP facility of the University of Poitiers for NanoUP-LC and mass spectrometry study.
This work was supported by La Ligue Contre le Cancer Grand Ouest (Comités de la Vienne, Deux-Sèvres), and Retina France. S.F. holds a PhD fellowship from the French Ministry for Education and Science.
Authorship
Contribution: S.F. performed the research, analyzed results, made the figures, and contributed to writing the manuscript; A.M. and M.P. performed the research and analyzed results; J.C., J.T., and C.S. performed experiments, analyzed results, and made the figures; C.K. and S.M.K. performed experiments, analyzed results, and contributed to writing the manuscript; O.B. initiated and designed the research project, analyzed results, and wrote the manuscript; and all authors contributed to the design and analysis of experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Omar Benzakour, Institut de Physiologie et Biologie Cellulaires, Centre National de la Recherche Scientifique FRE 3511, Université de Poitiers, Bât. B36, 1 rue Georges Bonnet-BP 633, 86022 Poitiers Cedex, France; e-mail: omar.benzakour@univ-poitiers.fr.