Abstract
Myeloproliferative neoplasms (MPNs) are the most common cause of Budd-Chiari syndrome (BCS) and nonmalignant, noncirrhotic portal vein thrombosis (PVT). In this meta-analysis, we determined the prevalence of MPNs and their subtypes as well as JAK2V617F and its diagnostic role in these uncommon disorders. MEDLINE and EMBASE databases were searched. Prevalence of MPNs, JAK2V617F, and MPN subtypes were calculated using a random-effects model. A total of 1062 BCS and 855 PVT patients were included. In BCS, mean prevalence of MPNs and JAK2V617F was 40.9% (95% CI, 32.9%-49.5%) and 41.1% (95% CI, 32.3%-50.6%), respectively. In PVT, mean prevalence of MPNs and JAK2V617F was 31.5% (95% CI, 25.1%-38.8%) and 27.7% (95% CI, 20.8%-35.8%), respectively. JAK2V617F and MPNs were more frequent in BCS compared with PVT (P = .03 and P = .09, respectively). Polycythemia vera was more prevalent in BCS than in PVT (P = .001). JAK2V617F screening in splanchnic vein thrombosis (SVT) patients without typical hematologic MPN features identified MPN in 17.1% and 15.4% of screened BCS and PVT patients, respectively. These results demonstrate a high prevalence of MPNs and JAK2V617F in SVT patients and show differences in underlying etiology between these disorders. Furthermore, these results validate routine inclusion of JAK2V617F in the diagnostic workup of SVT patients.
Introduction
Splanchnic vein thrombosis (SVT) includes the Budd-Chiari syndrome (BCS) and portal vein thrombosis (PVT). Primary BCS is characterized by thrombosis of the hepatic veins and/or the suprahepatic inferior vena cava, resulting in obstruction of the hepatic venous outflow tract.1 A distinct disorder that also includes the liver vasculature is PVT, which often occurs in association with local factors, such as liver cirrhosis or malignancy.2,3 PVT in the absence of liver cirrhosis or local malignancy is less frequently encountered and shows a considerable overlap in etiology with primary BCS. In this meta-analysis, we will focus exclusively on primary BCS and nonmalignant, noncirrhotic PVT.
Philadelphia-negative myeloproliferative neoplasms (MPNs) are the most frequent underlying prothrombotic factor in BCS and PVT, with a reported prevalence of 30%-50%4-9 and 15%-30%,2,6,10-12 respectively. Peripheral blood cell counts often remain within a normal range because of portal hypertension and its sequelae (splenomegaly, hemodilution, and iron deficiency). Despite suggestive features of an MPN, fulfillment of usual diagnostic criteria can therefore often be lacking, which is a notorious problem in MPN diagnostics in these patients. The term occult MPN has been used in the literature for patients who lack these typical hematologic features of MPN but who harbor clear features of MPN, for example, by means of bone marrow (BM) biopsy findings and growth of erythroid colonies in the absence of exogenous erythropoietin, referred to as spontaneous endogenous erythroid colonies, both of which have several limitations.12-14 BM biopsy is invasive, and the distinction between MPN and reactive BM is not unambiguous. Endogenous erythroid colony assays are performed only in specialized centers, are difficult to standardize, and offer the possibility of false positives in nonclonal causes of erythrocytosis and healthy controls.15
The discovery of the JAK2V617F gain-of-function mutation in 2005, found in 95% of patients with polycythemia vera (PV) and in ∼ 50% of patients with essential thrombocythemia (ET) and myelofibrosis (MF), represented a crucial advance in the diagnostic approach to MPNs.16-19 The close relationship between MPNs and BCS and PVT was confirmed by the high frequency of JAK2V617F among these patients, present in 30%-45%4,9,20 and 17%-35%,11,20,21 respectively. Interestingly, JAK2V617F screening offered a new diagnostic tool to detect these so-called occult MPNs in SVT patients, as this mutation was frequently demonstrated in SVT patients without characteristic elevated peripheral blood counts.22 JAK2V617F screening has since become part of the standard diagnostic workup in SVT.
Other advances in the field of MPNs were the identification of the MPL515 mutations in the thrombopoietin receptor gene in ∼ 5% and 10% of patients with JAK2V617F-negative ET and MF, respectively, and JAK2 exon 12 mutations in < 5% of JAK2V617F-negative PV patients.23-27 Both mutations have been described in small numbers of SVT patients, but their clinical relevance has not yet been fully clarified.28,29
The aims of this study were: (1) to assess the prevalence of MPNs and JAK2V617F in BCS and PVT patients; (2) to determine the frequency of MPN subtypes in BCS and PVT patients; (3) to determine JAK2V617F prevalence in BCS and PVT patients without typical hematologic features of MPN; and (4) to evaluate the clinical relevance of the MPL and JAK2 exon 12 mutations in BCS and PVT patients.
Methods
Search strategy and selection criteria
One of the authors (J.H.S.) searched Ovid MEDLINE and EMBASE from 1980 to August 1, 2011. The search strategy was restricted to published data and the English language using the subject headings presented in the Appendix. The search was supplemented by manually reviewing the reference list of retrieved articles and relevant reviews. Titles and abstracts of retrieved citations were screened, and potentially suitable studies were read in full by J.H.S. and F.W.G.L. Studies were selected when the following criteria were met: (1) patients were diagnosed with primary BCS or noncirrhotic, nonmalignant PVT, or patients with an underlying malignancy or cirrhosis were explicitly mentioned; (2) information on MPNs and/or JAK2V617F, JAK2 exon 12, or MPL515 was provided; (3) the cohort consisted of patients in which patients with established MPNs or other thrombophilic factors were not excluded; (4) SVT was subdivided in BCS and PVT; and (5) a minimum of 10 patients were included. Disagreements were resolved after discussion or after having collected the opinion of a third reviewer (H.L.A.J.).
Data extraction
J.H.S. extracted data on each selected study (year of publication, study design, demographics, criteria for diagnosing MPNs, number of patients included). Patients with BCS in the presence of a malignancy and PVT patients with a malignancy or cirrhosis were excluded from the analysis. Patients with combined BCS and PVT were classified as BCS according to common practice.4 MPNs were defined according to the diagnostic criteria used in the included studies. Different diagnostic criteria, mostly World Health Organization or Polycythemia Vera Study Group criteria, were used in the various studies. JAK2V617F, being discovered in 2005, was only reported in studies published after this date and was almost invariably presented as a separate entity, rather than being integrated in MPN workup as is now customary. We therefore extended MPN workup of these studies as follows. JAK2V617F was considered pathognomonic for MPN, and patients whodid not meet the diagnostic criteria for MPN but were found JAK2V617F-positive were classified as MPN. MPN subtypes were classified according to the diagnostic criteria used in each study. If high clinical suspicion based on typical hematologic features (ie, clinical, laboratory and/or morphological) of MPN existed in patients with JAK2V617F, but insufficient criteria for a specific subtype was met, the patient was classified as MPN unclassifiable. If JAK2V617F was present, but clinical, laboratory and/or morphologic data were insufficiently collected, patients were designated as solitary JAK2V617F positive MPN. Studies that did not report on JAK2V617F were only included in the MPN subtype analysis and not in the MPN prevalence analysis, as this would result in an underestimation of MPN prevalence. Corresponding authors were contacted in case essential data were not mentioned, with a reminder sent after 2 weeks.
Statistical analysis
Weighted mean proportion and 95% confidence intervals (CI) of MPNs, JAK2V617F, and MPN subtypes prevalence were calculated using a random effects model. Differences in prevalence were calculated by means of Pearson χ2. All statistical tests were 2-sided, and P < .05 was considered statistically significant. Statistical heterogeneity was evaluated using the I2 statistic, which describes the percentage of variation across studies that is the result of heterogeneity rather than chance, with P < .05 representing statistically significant heterogeneity. If heterogeneity was present, the analyses were repeated, removing one study a time to identify the source of heterogeneity. All analyses were performed with Comprehensive Meta Analysis Version 2.2 for Windows (Biostat), and the overall effects are presented as event rates with 95% CI.
Results
Study identification and selection
We identified 822 potentially relevant publications: 256 from MEDLINE and 566 from EMBASE. A total of 109 studies were duplicate, and 665 studies were excluded after title and abstracts screening according to predefined inclusion criteria. The remaining studies were retrieved in full for detailed evaluation. Five additional studies were identified from reference lists. Figure 1 shows the study selection process.
Of the 53 retrieved studies, 21 were excluded because of the following reasons: in 2 studies, patients with malignancies or liver cirrhosis were not excluded or explicitly mentioned30,31 ; 5 studies were based on selected cohorts in which patients with established MPNs or other thrombophilic factors were excluded32-36 ; one study did not differentiate SVT into BCS and PVT37 ; in 4 studies, MPN criteria were not acceptable or unclear14,38-40 ; and 2 studies included < 10 patients.29,41 In addition, 7 studies contained duplicate data.42-48 This resulted in 32 studies eligible for inclusion.
Study characteristics and quality
Tables 1 and 2 summarize the characteristics of the included studies for BCS and PVT, respectively. Study size ranged between 10 and 237 patients. Nineteen studies, including 1062 patients, reported on MPNs and/or the JAK2V617F mutation in BCS patients. Fifteen studies, including 855 patients, reported on MPNs and/or the JAK2V617F mutation in PVT patients. Three studies, including 268 patients, reported on JAK2 exon 12 mutations.20,28,49 Two studies, including 305 patients, reported on MPL515 mutations.20,28 Five studies included a healthy control population21,47,50-52 ; all other studies were essentially retrospective cohort studies. Twenty of these studies enrolled patients consecutively.2,4,5,7-9,11,12,21,28,52-61 Studied populations partly overlapped in 9 publications (ie, to some extent duplication of patients).2,5-7,9,14,20,47,62
Baseline characteristics of studies, including BCS patients
| Reference . | Y . | Design . | Male/female . | Median age, y (range) . | Median follow-up, mo (range) . | MPN criteria . | MPN . | JAK2V617F . | Classification (PV/ET/MF/U/solitaryJAK2)* . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||||||||
| Smira et al58 | 2010 | RC | NA | NA | NA | NA | NA | NA | 14/20 | 70 | NA |
| Zahn et al61 | 2010 | RC | 4/16 | 34 (14-60)† | NA | BM if MPN was suspected | 6/20 | 30 | NA | NA | 4/1/0/1/0 |
| Darwish Murad et al4 | 2009 | RC | 70/93 | 38 (16-83) | 17 (0.1-31) | WHO 2001, BM in majority of patients | 56/103 | 39 | 35/121 | 29 | 27/9/2/15/3 |
| Xavier et al60 | 2009 | RC | 11/20 | 33 (17-50) | 51 (1-104) | WHO 2001, BM if MPN was suspected | 8/31 | 26 | 8/31 | 26 | 4/2/0/0/2 |
| Rajani et al8 | 2009 | RC | 19/24 | 40 (4-80) | 32 (0.5-192) | BM in 79% of patients | 14/36 | 39 | NA | NA | 8/6/0/0 |
| Kiladjian et al20 | 2008 | RC | 69/35 | 36 (IQR 27-46) | 47 (range NA) | BM in nearly all patients | 47/104 | 45 | 47/104 | 45 | 17/3/0/27/0 |
| Colaizzo et al53 | 2008 | RC | 9/23 | 35 (14-66) | NA | WHO criteria 2001 | 17/32 | 53 | 11/32 | 34 | 4/1/9/2/1 |
| Uskudar et al59 | 2008 | RC | 40/35 | 34 (14-72)† | 18 (1-30) | BM if MPN was suspected | 6/72 | 8 | NA | NA | 5/1/0/0/0 |
| DeStefano et al63 | 2007 | RC | 4/11 | NA | 48 (24-108)‡ | PVSG 2000 | 5/15 | 33 | 5/15 | 33 | 1/3/0/0/1 |
| Smalberg et al9 | 2006 | RC | 14/26 | 28 (18-53) | 7.1 ± 6.9† | WHO 2001, BM in majority of patients | 13/40 | 33 | 7/17 | 41 | 6/6/0/1/0 |
| Patel et al51 | 2006 | RC | 15/26 | 36 ± 13.3 | 49 (8-87)§ | BM in all patients | 27/55 | 49 | 24/41 | 59 | 6/8/0/14/0 |
| Primignani et al52 | 2006 | RC | 8/12 | 33 (19-72) | NA | WHO 2001, based on BM only | 9/20 | 45 | 8/20 | 40 | 3/3/0/3/0 |
| Eapen et al62 | 2006 | RC | 22/39 | 36 (16-77) | 52 (0-181) | Not specified | 17/61 | 28 | NA | NA | 7/6/1/3/0 |
| Khuroo et al54 | 2005 | RC | 17/23 | 27 ± 7.3† | NA | BM if MPN was suspected | 4/40 | 10 | NA | NA | 1/3/0/0/0 |
| Darwish Murad et al5 | 2004 | RC | 78/159 | 35 (13-76) | 44 (0-203) | BM if MPN was suspected | 54/237 | 23 | NA | NA | 45/9/0/0/0 |
| Attwell et al74 | 2004 | RC | 7/15 | 24 (18-68)† | NA | BM if MPN was suspected | 11/22 | 50 | NA | NA | 8/3/0/0/0 |
| Janssen et al47 | 2000 | CC | 16/27 | 40 (19-60) | NA | BM if MPN was suspected | 12/43 | 28 | NA | NA | 10/1/0/1/0 |
| Denninger et al6 | 2000 | RC | NA | NA | NA | BM if MPN was suspected | 12/32 | 38 | NA | NA | 12/0/0/4/0 |
| Mahmoud et al7 | 1996 | RC | 17/27 | 37 (19-60)† | NA | BM if MPN was suspected | 17/42 | 40 | NA | NA | 11/5/1/0/0 |
| Reference . | Y . | Design . | Male/female . | Median age, y (range) . | Median follow-up, mo (range) . | MPN criteria . | MPN . | JAK2V617F . | Classification (PV/ET/MF/U/solitaryJAK2)* . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||||||||
| Smira et al58 | 2010 | RC | NA | NA | NA | NA | NA | NA | 14/20 | 70 | NA |
| Zahn et al61 | 2010 | RC | 4/16 | 34 (14-60)† | NA | BM if MPN was suspected | 6/20 | 30 | NA | NA | 4/1/0/1/0 |
| Darwish Murad et al4 | 2009 | RC | 70/93 | 38 (16-83) | 17 (0.1-31) | WHO 2001, BM in majority of patients | 56/103 | 39 | 35/121 | 29 | 27/9/2/15/3 |
| Xavier et al60 | 2009 | RC | 11/20 | 33 (17-50) | 51 (1-104) | WHO 2001, BM if MPN was suspected | 8/31 | 26 | 8/31 | 26 | 4/2/0/0/2 |
| Rajani et al8 | 2009 | RC | 19/24 | 40 (4-80) | 32 (0.5-192) | BM in 79% of patients | 14/36 | 39 | NA | NA | 8/6/0/0 |
| Kiladjian et al20 | 2008 | RC | 69/35 | 36 (IQR 27-46) | 47 (range NA) | BM in nearly all patients | 47/104 | 45 | 47/104 | 45 | 17/3/0/27/0 |
| Colaizzo et al53 | 2008 | RC | 9/23 | 35 (14-66) | NA | WHO criteria 2001 | 17/32 | 53 | 11/32 | 34 | 4/1/9/2/1 |
| Uskudar et al59 | 2008 | RC | 40/35 | 34 (14-72)† | 18 (1-30) | BM if MPN was suspected | 6/72 | 8 | NA | NA | 5/1/0/0/0 |
| DeStefano et al63 | 2007 | RC | 4/11 | NA | 48 (24-108)‡ | PVSG 2000 | 5/15 | 33 | 5/15 | 33 | 1/3/0/0/1 |
| Smalberg et al9 | 2006 | RC | 14/26 | 28 (18-53) | 7.1 ± 6.9† | WHO 2001, BM in majority of patients | 13/40 | 33 | 7/17 | 41 | 6/6/0/1/0 |
| Patel et al51 | 2006 | RC | 15/26 | 36 ± 13.3 | 49 (8-87)§ | BM in all patients | 27/55 | 49 | 24/41 | 59 | 6/8/0/14/0 |
| Primignani et al52 | 2006 | RC | 8/12 | 33 (19-72) | NA | WHO 2001, based on BM only | 9/20 | 45 | 8/20 | 40 | 3/3/0/3/0 |
| Eapen et al62 | 2006 | RC | 22/39 | 36 (16-77) | 52 (0-181) | Not specified | 17/61 | 28 | NA | NA | 7/6/1/3/0 |
| Khuroo et al54 | 2005 | RC | 17/23 | 27 ± 7.3† | NA | BM if MPN was suspected | 4/40 | 10 | NA | NA | 1/3/0/0/0 |
| Darwish Murad et al5 | 2004 | RC | 78/159 | 35 (13-76) | 44 (0-203) | BM if MPN was suspected | 54/237 | 23 | NA | NA | 45/9/0/0/0 |
| Attwell et al74 | 2004 | RC | 7/15 | 24 (18-68)† | NA | BM if MPN was suspected | 11/22 | 50 | NA | NA | 8/3/0/0/0 |
| Janssen et al47 | 2000 | CC | 16/27 | 40 (19-60) | NA | BM if MPN was suspected | 12/43 | 28 | NA | NA | 10/1/0/1/0 |
| Denninger et al6 | 2000 | RC | NA | NA | NA | BM if MPN was suspected | 12/32 | 38 | NA | NA | 12/0/0/4/0 |
| Mahmoud et al7 | 1996 | RC | 17/27 | 37 (19-60)† | NA | BM if MPN was suspected | 17/42 | 40 | NA | NA | 11/5/1/0/0 |
RC indicates retrospective cohort; CC, case-control; U, unclassifiable; solitaryJAK2, solitary JAK2-positive; NA, not available; IQR, interquartile range; and BM, bone marrow biopsy.
MPNs that became overt during follow-up were included in subtype analysis.
Mean age/follow-up (range) or ± SD.
Median follow-up of patients with JAK2V617F-positive MPNs without elevated blood counts.
Median time of diagnosis to overt MPNs.
Baseline characteristics of studies including portal vein thrombosis patients
| Reference . | Y . | Design . | Male/female . | Median age, y (range) . | Median follow-up, mo, (range) . | MPN criteria . | MPNs . | JAK2V617F . | Classification (PV/ET/MF/U/solitaryJAK2)* . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||||||||
| Hoekstra et al64 | 2011 | RC | 13/31 | 48 (18-79) | 70 (5-252) | WHO 2008 criteria | NA | NA | NA | NA | 14/12/7/11/0 |
| Rajani et al57 | 2010 | RC | 80/93 | 57 (15-94) | 30 (0-116) | Not specified, BM in majority of patients | 15/89 | 17 | NA | NA | 10/4/0/0/0§ |
| Orr et al56 | 2010 | RC | 14/21 | 43 (18-72) | 51 (10-300) | WHO 2001, BM if MPN was suspected | 6/35 | 17 | 16/35 | 46 | 2/3/1/1/9§ |
| Plessier et al11 | 2010 | RC | 50/52 | 48 (16-84) | 20 (0-75) | WHO 2001, BM in majority of patients | 17/102 | 17 | 14/82 | 17 | 3/11/3/2/2 |
| Xavier et al60 | 2009 | RC | 40/37 | 42 (17-74) | 51 (1-104) | WHO 2001, BM if MPN was suspected | 3/76 | 4 | 15/76 | 20 | 1/3/2/1/8 |
| Kiladjian et al20 | 2008 | RC | 77/60 | 42 (IQR 30-57) | 66 (range NA) | Not specified, BM in nearly all patients | 48/137 | 28 | 47/137 | 34 | 14/8/3/23/0 |
| Bayraktar et al10 | 2008 | RC | 9/16 | 45 (24-73)† | NA | WHO, BM if MPN was suspected | 6/25 | 24 | 6/25 | 24 | 3/2/1/0/5 |
| DeStefano et al63 | 2007 | RC | 27/31 | NA | 48 (24-108)‡ | PVSG 2000 | 8/58 | 14 | 24/58 | 41 | 4/5/0/0/15 |
| McMahon et al50 | 2007 | RC | 9/1 | NA | NA | Not specified | 0/10 | 0 | 1/10 | 10 | 0/0/0/0/1 |
| Colaizzo et al21 | 2007 | RC | 44/55 | 41 (10-85) | 41 (3-114) | WHO 2001 | 9/99 | 9 | 17/99 | 17 | 3/5/5/2/5§ |
| Primignani et al52 | 2006 | RC | 29/44 | 42 (13-66) | NA | WHO 2001, based on BM only | 31/55 | 56 | 26/73 | 36 | 5/14/5/9/0 |
| Kocher et al55 | 2005 | RC | 10/10 | 51 (17-83) | 21 (2-61) | Not specified, BM if MPN was suspected | 6/20 | 30 | NA | NA | 2/4/0/0/0 |
| Janssen et al2 | 2001 | RC | NA | NA | 3.9 (0.1-13.1)† | Not specified, BM if MPN was suspected | 22/82 | 27 | NA | NA | 12/2/5/2/0§ |
| Denninger et al6 | 2000 | RC | NA | NA | NA | Not specified, BM if MPN was suspected | 5/36 | 14 | NA | NA | 5/0/0/6/0 |
| Valla et al12 | 1988 | RC | 14/17 | NA | NA | Not specified, BM if MPN was suspected | 7/31 | 23 | NA | NA | 2/1/2/6/0 |
| Reference . | Y . | Design . | Male/female . | Median age, y (range) . | Median follow-up, mo, (range) . | MPN criteria . | MPNs . | JAK2V617F . | Classification (PV/ET/MF/U/solitaryJAK2)* . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||||||||
| Hoekstra et al64 | 2011 | RC | 13/31 | 48 (18-79) | 70 (5-252) | WHO 2008 criteria | NA | NA | NA | NA | 14/12/7/11/0 |
| Rajani et al57 | 2010 | RC | 80/93 | 57 (15-94) | 30 (0-116) | Not specified, BM in majority of patients | 15/89 | 17 | NA | NA | 10/4/0/0/0§ |
| Orr et al56 | 2010 | RC | 14/21 | 43 (18-72) | 51 (10-300) | WHO 2001, BM if MPN was suspected | 6/35 | 17 | 16/35 | 46 | 2/3/1/1/9§ |
| Plessier et al11 | 2010 | RC | 50/52 | 48 (16-84) | 20 (0-75) | WHO 2001, BM in majority of patients | 17/102 | 17 | 14/82 | 17 | 3/11/3/2/2 |
| Xavier et al60 | 2009 | RC | 40/37 | 42 (17-74) | 51 (1-104) | WHO 2001, BM if MPN was suspected | 3/76 | 4 | 15/76 | 20 | 1/3/2/1/8 |
| Kiladjian et al20 | 2008 | RC | 77/60 | 42 (IQR 30-57) | 66 (range NA) | Not specified, BM in nearly all patients | 48/137 | 28 | 47/137 | 34 | 14/8/3/23/0 |
| Bayraktar et al10 | 2008 | RC | 9/16 | 45 (24-73)† | NA | WHO, BM if MPN was suspected | 6/25 | 24 | 6/25 | 24 | 3/2/1/0/5 |
| DeStefano et al63 | 2007 | RC | 27/31 | NA | 48 (24-108)‡ | PVSG 2000 | 8/58 | 14 | 24/58 | 41 | 4/5/0/0/15 |
| McMahon et al50 | 2007 | RC | 9/1 | NA | NA | Not specified | 0/10 | 0 | 1/10 | 10 | 0/0/0/0/1 |
| Colaizzo et al21 | 2007 | RC | 44/55 | 41 (10-85) | 41 (3-114) | WHO 2001 | 9/99 | 9 | 17/99 | 17 | 3/5/5/2/5§ |
| Primignani et al52 | 2006 | RC | 29/44 | 42 (13-66) | NA | WHO 2001, based on BM only | 31/55 | 56 | 26/73 | 36 | 5/14/5/9/0 |
| Kocher et al55 | 2005 | RC | 10/10 | 51 (17-83) | 21 (2-61) | Not specified, BM if MPN was suspected | 6/20 | 30 | NA | NA | 2/4/0/0/0 |
| Janssen et al2 | 2001 | RC | NA | NA | 3.9 (0.1-13.1)† | Not specified, BM if MPN was suspected | 22/82 | 27 | NA | NA | 12/2/5/2/0§ |
| Denninger et al6 | 2000 | RC | NA | NA | NA | Not specified, BM if MPN was suspected | 5/36 | 14 | NA | NA | 5/0/0/6/0 |
| Valla et al12 | 1988 | RC | 14/17 | NA | NA | Not specified, BM if MPN was suspected | 7/31 | 23 | NA | NA | 2/1/2/6/0 |
RC indicates retrospective cohort; CC, case-control; U, unclassifiable; solitaryJAK2, solitary JAK2-positive; NA, not available; IQR, interquartile range; and BM, bone marrow biopsy.
MPNs that became overt during follow-up were included in subtype analysis.
Mean age/follow-up (range) was reported.
Median follow-up of patients with JAK2V617F-positive MPNs without elevated blood counts.
In each of these studies, also one patient with chronic myeloid leukemia was reported.
MPNs and JAK2V617F in BCS
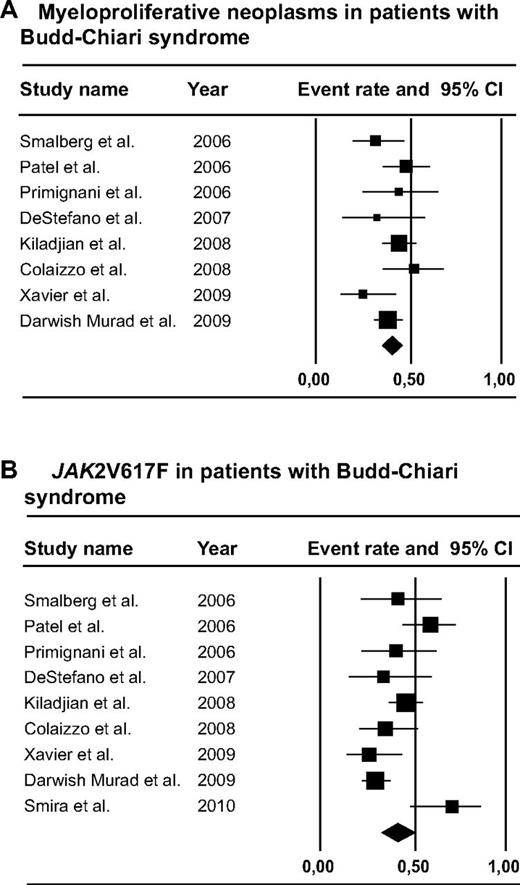
A total of 1062 BCS patients were included in the analysis (Figure 2). Of these patients, 440 underwent a complete diagnostic workup for MPN, including clinical, laboratory, and/or morphologic features of MPN as well as JAK2V617F mutation analysis. MPN was present in 40.9% (95% CI, 32.9%-49.5%) of these patients. Of the MPN patients, 80.3% were JAK2V617F-positive (95% CI, 63.5%-90.5%). The JAK2V617F mutation was present in 159 of 401 tested patients, for a mean prevalence of 41.1% (95% CI, 32.3%-50.6%). JAK2V617F screening in patients without typical hematologic features of MPN yielded diagnosis of MPN in 17.1% (95% CI, 7.9%-33.3%). Distribution of MPN subtypes was as follows: PV, ET, MF, unclassifiable MPNs, and solitary JAK2V617F-positive MPNs in 52.9% (95% CI, 42.2%-63.4%), 24.6% (95% CI, 18.0%-32.5%), 6.7% (95% CI, 3.7%-11.9%), 17.0% (95% CI, 9.8%-27.9%), and 6.5% (95% CI, 2.4%-16.3%), respectively (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Forest plots. (A) MPNs in patients with BCS. (B) JAK2V617F in patients with BCS.
Forest plots. (A) MPNs in patients with BCS. (B) JAK2V617F in patients with BCS.
Four studies made a distinction between diagnosis of MPN before or simultaneous to BCS, in which 13 of 50 patients were diagnosed with MPN before BCS, whereas BCS was the presenting symptom of MPN in 37 of 50 patients.7,9,53,60 Follow-up of JAK2V617F-positive MPNs without typical hematologic MPN features was provided in 3 publications, in which 11 of 28 patients (41%) developed characteristic laboratory or morphologic features of MPN, ranging from 0.7 to 7 years after diagnosis of BCS.51,60,63
MPNs and JAK2V617F in PVT
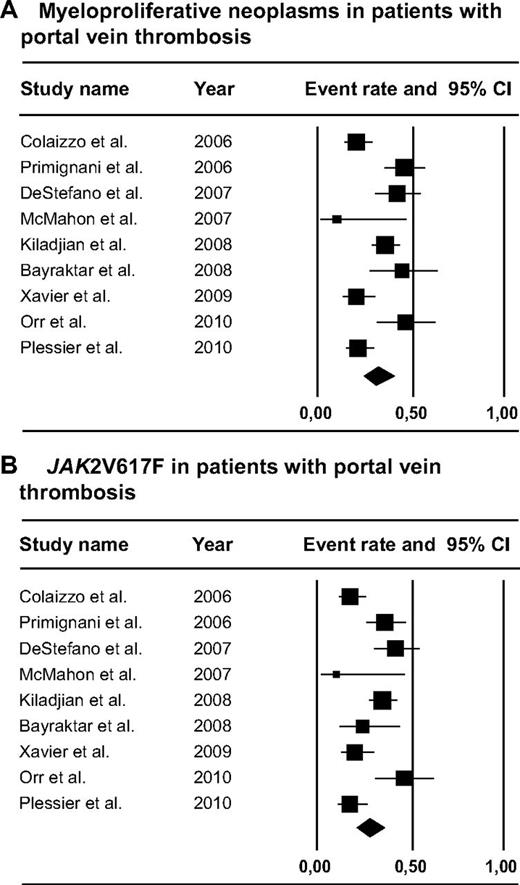
A total of 855 PVT patients were included in the analysis (Figure 3). MPNs were present in 188 of 615 or 31.5% (95% CI, 25.1%-38.8%) of the patients who underwent complete diagnostic workup for MPNs, including clinical, laboratory, and/or morphologic features of MPN as well as JAK2V617F mutation analysis. Of the MPN patients, 86.6% were JAK2V617F-positive (95% CI, 73.1%-93.9%). The JAK2V617F mutation was present in 166 of 595 tested patients, for a mean prevalence of 27.7% (95% CI, 20.8%-35.8%). JAK2V617F mutation analysis yielded diagnosis of MPN in 15.4% (95% CI, 7.9%-33.3%) of screened PVT patients without characteristic hematologic features of MPNs, which would otherwise have been undiagnosed. Distribution of MPN subtypes was as follows: PV, ET, MF, unclassifiable MPNs, and solitary JAK2V617F-positive MPNs in 27.5% (95% CI, 19.0%-38.1%), 26.2% (95% CI, 19.1%-34.8%), 12.8% (95% CI, 8.0%-19.9%), 17.7% (95% CI, 9.9%-29.7%), and 24.0% (95% CI, 11.5%-43.3%), respectively (supplemental Figure 1).
Forest plots. (A) MPNs in patients with PVT. (B) JAK2V617F in patients with PVT.
Forest plots. (A) MPNs in patients with PVT. (B) JAK2V617F in patients with PVT.
Five studies differentiated between diagnosis of MPN before or simultaneous to PVT diagnosis, showing that 17 of 64 patients were diagnosed with MPN before PVT, whereas PVT was the presenting symptom of MPN in 47 of 64 patients.10,14,56,60,64 Follow-up of JAK2V617F-positive MPNs without typical MPN features counts was provided in 4 publications in which 6 of 48 patients (13%) developed characteristic laboratory or morphologic features of MPN, ranging from 1-10 years after diagnosis of PVT.21,56,60,63 One study described the long-term follow-up of 44 PVT patients with an underlying MPN.64 Five PV and 2 ET patients developed secondary MF, 3 patients with MF progressed to end-stage MF, and 4 patients developed acute myeloid leukemia after a median period of 9.7 years (range, 1-17 years) after MPN diagnosis. A total of 29% and 18% of the deaths in this cohort were attributable to end-stage MF and progression to acute myeloid leukemia, respectively.
JAK2 exon 12 and MPL515 mutations in SVT
A total of 268 SVT patients (ratio BCS/PVT unknown) were tested for JAK2 exon 12 and 305 for MPL515 mutations. Three of these patients were found to carry MPLW515K mutation. The JAK2 exon 12 mutation was not present in any of these patients.
Differences between BCS and PVT
Prevalence of JAK2V617F and MPNs was higher in BCS than in PVT (P = .03 and P = .09, respectively). With regards to the subtype analysis, prevalence of PV and MF was higher in BCS than in PVT patients (P = .001 and P = .09, respectively). Prevalence of solitary JAK2V617F-positive MPNs was higher in PVT compared with BCS (P = .03). There was no difference between the prevalence of ET and MPNs unclassifiable (P = .77 and P = .92, respectively). There was no difference in identification rate of MPNs without typical features by means of JAK2V617F between the 2 disorders (17.1% vs 15.4%, P = .68). All analyses were repeated, including only publications since 2005 and excluding papers with potentially duplicated inclusion of patients, which showed the same results (data not shown).
I2 and heterogeneity among studies
A considerable heterogeneity among the studies was observed (P < .05). We therefore performed an additional analysis in which we excluded one study per analysis. This analysis showed that no single study significantly affected the point estimate of MPNs, JAK2V617F, and its subtypes in both BCS and PVT.
Discussion
In this meta-analysis, we assessed the role of MPNs in the etiology of primary BCS and nonmalignant, noncirrhotic PVT. The results showed a higher prevalence of MPNs and JAK2V617F in BCS compared with PVT patients. Interestingly, our results indicate a difference in the distribution of underlying MPN subtype between BCS and PVT patients, PV being the most frequent MPN in BCS. Finally, MPL515 mutations were present in < 1% of BCS and PVT series, whereas JAK2 exon 12 mutations have never been published so far in SVT patients.
Two meta-analyses have previously evaluated the impact of the JAK2V617F mutation in SVT patients. In 2009, Dentali et al assessed the role of JAK2V617F in patients with various venous thrombosis, including SVT, deep vein thrombosis of the lower extremities or pulmonary embolism, cerebral vein thrombosis, and retinal vein thrombosis.65 In this study, a remarkable high prevalence of JAK2V617F in SVT was reported, whereas its prevalence in other forms of VTE was similar to that of the general population. SVT was not subdivided into BCS and PVT, which impedes comparison of MPNs and JAK2V617F prevalence between the 2 disorders. Qi et al calculated the prevalence of JAK2V617F in BCS and PVT separately and assessed its prevalence after exclusion of cases with preexisting MPNs.66 In contrast to those previously published studies, we set out to provide a complete overview of MPNs in the etiology of BCS and PVT. This included assessment of the prevalence of MPNs and JAK2V617F as well as the prevalence of MPN subtypes. In addition, we have compared BCS and PVT for each of these variables, as it is increasingly recognized that, despite several similarities, risk profiles are different between these patients.67 To achieve this goal, we have assessed all the publications regarding MPNs in SVT since 1980.
The results of this meta-analysis indicate a high prevalence of MPNs in patients with SVT. The strong relation between MPNs and SVT is confirmed by the high prevalence of JAK2V617F in these patients. Interestingly, JAK2V617F and MPNs were more prevalent in BCS compared with PVT patients, the latter showing a statistical trend rather than a significant difference. This difference may be partially explained by the more prominent role of local risk factors, such as focal inflammatory lesions and injury to the portal venous system, in the development of PVT.68 This might contribute to the relatively limited role of general prothrombotic conditions reported in the etiology of PVT. Why MPNs and JAK2V617F are so strongly related to thrombosis of the splanchnic veins remains an unresolved issue. Further research is needed to identify associated factors that could be involved in the pathogenesis of thrombosis at these specific sites. In this respect, it has been speculated that endothelial cells of the splanchnic veins may interact with activated platelets and/or leukocytes and increased microparticles, which are characteristic features of MPNs.69 In addition, these endothelial cells have been shown to carry the JAK2V617F mutation and could be part of the malignant process.70
We observed a marked difference between BCS and PVT patients regarding the distribution of MPN subtypes. PV was clearly more common in BCS compared with PVT. The prothrombotic effect of high hematocrit values in PV is well established.71 Under low-shear conditions, such as in the venous circulation, a high hematocrit has a more important impact on blood viscosity and causes a major disturbance to blood flow.72,73 This mechanism may be mediated by the interaction between adhesion molecules and red blood cells. Wautier et al described an increased adhesiveness of red blood cells in PV to human umbilical vein endothelial cells and elegantly showed that adhesion was inversely related to increasing shear stress (ie, adhesion proved particularly increased at low shear rates).9 It is possible that variability in the expression of these molecules along the vascular tree along with differences in flow conditions might contribute to the site specificity of thrombosis, as suggested by these authors.9 Indeed, the low-flow state in the hepatic veins compared with the portal venous system may participate in the higher frequency of PV in BCS. We also observed a statistical trend toward increased frequency of MF in PVT compared with BCS. Such difference could be the result of the frequent presence of splenomegaly in MF, which may lead to external compression of the portal venous system and subsequent stasis of blood flow. Finally, solitary JAK2V617F-positive MPNs were more frequent in PVT than in BCS patients. These are new findings that deserve further evaluation in future studies.
This meta-analysis, for the first time, systematically assessed the diagnostic yield of JAK2V617F screening in SVT patients without typical hematologic MPN features. JAK2V617F screening identified MPN in 17.1% and 15.4% of these BCS and PVT patients, respectively, which would have remained undetected before the JAK2V617F era. JAK2V617F was associated with subsequent development of MPNs with typical hematologic MPN features in 41% and 14% of these BCS and PVT patients, respectively. These findings clearly substantiate inclusion of JAK2V617F in the routine diagnostic workup of all SVT patients, regardless of the absence of MPN hallmarks, such as elevated peripheral blood cell counts. Whether MPN specific treatment should be initiated in these patients, such as cytoreductive therapies or addition of aspirin to oral anticoagulant treatment, is a question that remains to be answered. One study described the long-term outcome of PVT patients with an underlying MPN.64 Twenty-nine and 18% of the deaths in this cohort were attributable to end-stage MF and progression to acute myeloid leukemia, respectively, indicating that risk of MPN progression is a clinical significant issue in these patients.
MPL515 mutations were reported in < 1% of SVT patients, whereas the JAK2 exon 12 could not be found at all. The JAK2 exon 12 mutation has been described only once in both a PVT and BCS patient, but this was a case study.29 These results indicate that both mutations are infrequent in SVT patients, in agreement with their low frequency in MPNs compared with the JAK2V617F mutation.23-27 We therefore conclude that, unlike JAK2V617F, screening for these mutations is dispensable in the routine diagnostic approach of SVT patients.
Our analysis has several potential limitations. First, because of the rarity of both diseases, only observational studies have been published and could be included in this analysis, with their inherent risks of bias. However, a prospective design for rare thrombotic manifestations as PVT and BCS is probably unachievable. Second, a considerable heterogeneity among the included studies was noticed. We therefore performed all analyses using a random-effects model, thereby accounting for between-study variance, next to within-study variance. In addition to the random-effects analysis, which generates a conservative estimate, we performed an analysis in which we excluded one study at a time to assess its individual impact on the results. This analysis showed that none of the included studies significantly affected the estimated prevalence of MPNs, JAK2V617F, and its subtypes in both BCS and PVT. Third, diagnostic criteria for MPNs were not similar across studies. Notably, BM biopsy was not always routinely performed, which may have resulted in an underestimation of the prevalence of MPNs. Because this applies to both BCS and PVT series, the effect on the comparison between these 2 groups is presumably small, if at all present. Lastly, since the discovery of JAK2V617F in 2005, an increase in larger and better quality studies was observed. We therefore repeated all analyses, including only publications from that time point. In addition, we excluded papers with potential overlap of patients. The same differences between BCS and PVT were observed.
In conclusion, this meta-analysis shows a high prevalence of MPNs and JAK2V617F in SVT patients. Prevalence of JAK2V617F and MPNs in BCS is higher compared with PVT, and differences in underlying MPN subtypes between these disorders exist. JAK2V617F screening identifies MPNs in patients without typical hematologic MPN features and should be included the routine diagnostic workup of SVT. On the contrary, JAK2 exon 12 and MPL515 mutations are extremely rare in SVT and should not be used in the routine diagnostic approach of SVT patients. Altogether, our results are in line with the advancing insight that, despite well-established similarities, marked differences in the etiology of BCS and PVT do exist.
Appendix: Medline search strategy
August 1, 2011
Database: Medline.
Limits: English, limits publication date January 1, 1980 to August 1, 2011:
Myeloproliferative disorders [Mesh]: 23 690
Myeloproliferative neoplasms: 2798
Janus kinase 2 [Mesh]: 2880
MPL protein, human [substance name]: 503
Colony-forming units assay: 13111
Budd-Chiari syndrome [Mesh]: 1539
Hepatic vein thrombosis: 2885
Hepatic venous thrombosis: 1544
Hepatic outflow obstruction: 295
Vascular liver disease: 11915
Thrombosis [Mesh] AND vena cava, inferior [Mesh]: 1784
Portal system [Mesh] AND thrombosis [Mesh]: 2535
Portal vein thrombosis: 3158
Portal venous thrombosis: 2015
Splanchnic vein thrombosis: 206
Splanchnic venous thrombosis: 163
Abdominal vein thrombosis: 2091
Abdominal venous thrombosis: 1587
6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18: 19 561
1 and 19: 214
2 and 19: 45
3 and 19: 57
4 and 19: 3
5 and 19: 12
20 or 21 or 22 or 23 or 24: 255
The search was supplemented by manually reviewing the reference list of retrieved articles.
The online version of this article contains a data supplement.
Authorship
Contribution: J.H.S. conceived and designed the study, collected, assembled, and interpreted the data, and wrote the manuscript; L.R.A. performed data analysis; J.-J.K. and D.C.V. interpreted the data and critically revised the article for important intellectual content; H.L.A.J. assisted in study selection and analysis of results and critically revised the article for important intellectual content; and F.W.G.L. designed the study, selected included studies, analyzed the results, and assisted with writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frank W. G. Leebeek, Department of Hematology, University Medical Center Rotterdam, Room L-438, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.