Abstract
Despite the rewarding results achieved in the treatment of Hodgkin lymphoma (HL), concerns have been raised regarding the long-term complications induced by therapy. Hence, the current challenge is to develop a new therapeutic strategy maintaining excellent patient outcome while reducing potentially life-threatening late adverse effects. Therefore, it would be beneficial to identify chemoresistant or refractory patients early during therapy for appropriate and timely escalation of treatment. Recently, compelling data have emerged on the prognostic role of interim [18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) performed early during the course of treatment to predict ultimate outcome, even proving superior to conventional prognostic factors. Several ongoing prospective trials are exploring the feasibility of treatment de-escalation strategies in patients with a negative interim PET, as well as therapy escalation in advanced-stage HL patients who have a positive interim PET result. In this article, the published reports on the contribution of interim PET to the design of ongoing response-adapted clinical trials are reviewed. Moreover, some of the unresolved issues revolving around the suboptimal positive predictive value of interim PET are addressed with an emphasis on the interpretation criteria. A final remark on the appropriate use of interim PET is also provided.
Introduction
Hodgkin lymphoma (HL) is a highly curable disease, with > 90% of patients becoming long-term survivors and 80% are deemed cured after a minimum follow-up of 6 years.1 These gratifying results are not only on account of high tumor chemosensitivity and radiosensitivity but also of a combination of evolving improvements in the staging accuracy, discovery of effective prognostic factors, adoption of tailored treatment strategies based on predefined risk groups, and optimization of radiation treatment (RT) planning.
The combination of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD), as first-line treatment, induces a durable remission in 75%-88% of the HL cases.2 However, for patients with primary refractory disease, or those experiencing relapse after the first complete remission (CR), the disease may become life-threatening. Indeed, 10%-15% of early stage and 20%-30% of the advanced-stage patients fail to achieve durable remissions, ultimately dying of resistant or recurrent HL.3 Hence, there remains an unfulfilled need for a valid means to predict the completeness of therapy response and ultimate patient outcome. Ideally before, or early during treatment, identification of a patient subset in which continuation of standard therapy would be ineffective is preferable, to be able to timely institute a more effective therapy to achieve CR. [18F]-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET), particularly, when integrated with computed tomography (PET/CT), yielded highly promising results as a surrogate test for determining tumor chemosensitivity and outcome,4-11 with a sensitivity and specificity ranging between 43%-100% and 67%-100%, respectively,12 even challenging the validity of International Prognostic Score.13
HL is among the most chemosensitive lymphoma entities, although the biologic mechanism for this phenomenon is unclear. A possible explanation could lie in the idiosyncratic tumor architecture. The tumor cells, the Reed-Sternberg and Hodgkin cells, accounting for < 1% of the total cell count of the neoplastic tissue, are surrounded by a functional network of non-neoplastic mononuclear bystander cells.14 The latter show considerably high glycolytic activity15 and are responsible in part for the high FDG uptake within the tumor tissue.16 Both chemokine production and metabolic activity of this microenvironment are apparently shut down after 2 courses of ABVD treatment in nearly 80% of patients.7,8,17,18 The paradoxical phenomenon of a persistent mass associated with no metabolically active tumor cells, termed as “metabolic complete remission” supports a high negative predictive value for interim-PET for predicting treatment outcome.19-21
End-of-therapy response assessment was one of the first clinical applications of FDG- PET imaging in oncology and by the end of the millennium several reports were published emphasizing its usefulness. In a recent meta-analysis, the pooled sensitivity and specificity of PET in distinguishing between different treatment outcomes in HL and diffuse large B-cell lymphoma were 84% and 90%, respectively.22 Different from other lymphoma subsets, HL is associated with a post-treatment residual mass that is encountered in up to 80% of patients, including those with CR.23,24 In this context, a PET-negative residual mass is considered to be unlikely to relapse. Since a seminal study by Jerusalem et al,25 the reports focused on the role of FDG-PET for the post-treatment evaluation of a residual mass in HL yielded a sensitivity and specificity of 43%-100%, and 67%-100%, respectively.22 In the case of a solitary persistent mass at completion of treatment, FDG-PET positivity was proposed to be the dominant determinant of pursuing consolidation RT.26 Finally, in the era of modern radiotherapy, for early-stage HL, extended fields that were originally developed for single-modality treatment have been replaced by conformal fields designed for combined modality treatments. Using fusion PET/CT images, the lymphoma volume determined on the staging and postchemotherapy PET/CT yields significantly more accurate gross tumor volumes for the involved nodes compared with those defined by CT alone.27,28 In summary, the growing number of HL survivors for all age subgroups over the past 40 years29 undoubtedly reflects the overall improvement in lymphoma management and treatment. More recently, the effective use of advanced imaging technology as a guidance tool for strategic management decisions may even further improve survival.
Interim response assessment: early-stage HL
Approximately one-third of patients diagnosed with HL present with limited-stage disease and have an anticipated cure rate of 90%-95% with the currently available first-line treatment options. Late effects of therapy, including the risk of developing second malignancies and cardiovascular disease, take center stage in the long-term follow-up of these patients. Hence, the emphasis has shifted toward treatment de-escalation to minimize toxicity and treatment-associated late complications while maintaining therapeutic efficacy. Nonetheless, it is not clear whether it is feasible to provide patients with an equally favorable outcome by reducing the number of treatment cycles or omitting RT. Four cycles of ABVD, followed by involved-field RT (IFRT), was regarded by many groups as the standard of care treatment for early-stage HL patients, irrespective of prognostic factors.30-32 Although chemotherapy alone may be a viable alternative management strategy, this option remains controversial among various groups.33-35 The results of the HD10 trial of the German Hodgkin Study Group (GHSG) in patients with early-stage HL and a favorable prognosis have recently demonstrated that treatment with 2 cycles of ABVD followed by 20 Gy of IFRT was as effective as, and less toxic than, 4 cycles of ABVD followed by 30 Gy of IFRT.36 Adverse events and acute toxic effects of treatment were most common in those patients who received 4 cycles of ABVD and 30 Gy of IFRT. Although not adapted by interim PET results, these data strongly suggest that abbreviated chemotherapy with ABVD followed by attenuated doses of radiotherapy is highly effective at eradicating limited-stage HL. However, overtreatment with RT and its attendant long-term adverse effects for a subset of patients still remain as a concern to many clinicians. In the pre-PET era, the National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group37 have shown that freedom from disease progression was superior in patients achieving a complete or unconfirmed complete remission after 2 cycles of ABVD (P = .007; 5-year survival estimates, 95% vs 81%) than those who did not after completing therapy with 2 more cycles with no ensuing RT. Interestingly, those with only a partial response after 2 cycles of treatment had a high likelihood of cure with the continuation of ABVD, resulting in a long-term disease control in 80% of the patients. In this group, the intriguing question would be whether or not interim PET/CT could have the ability to identify eventually relapsing patients who could be offered consolidative RT despite a full schedule chemotherapy protocol. For this particular clinical question, interim PET may be an attractive surrogate tool for response. However, the high rate of false-positive results (30%-50%) raises significant concerns6,12,38 on the basis of unjustifiably intensifying therapy in a subgroup of PET-positive patients. Unwarranted escalation to a significantly more toxic treatment is even more inappropriate in the younger population because of the increased risk of developing secondary leukemia and early menopause for women.39 Tissue biopsy may be helpful in many patients but does not resolve all challenges, particularly the question related to the curability of biopsy-positive cases as well as sampling errors. In this regard, dual-point PET scanning may be beneficial in reducing the false-positive findings without the need for an invasive approach.40 Escalation of therapy to BEACOPP based on a positive interim PET scan represents the underpinning of the design of various trials, including H10 trial by EORTC/GELA/ILL, CALGB 50604 and 50801 trials, and ECOG 2410 trial (Table 1). The first interim analysis of EORTC/GELA H10 study of early stage supradiaphragmatic HL inclusive of favorable and unfavorable risk categories reported on 894 of 1097 recruited patients. Using IHP criteria, interim PET was positive in 14% and 24% in the favorable and unfavorable categories, respectively. FDG-PET treatment adaptation was, thus, proven feasible in an intergroup randomized trial.49 There were 12 and 22 events in favorable and unfavorable groups, respectively, during a median follow-up of 1.1 years. On the conclusion that chemotherapy alone would be unlikely to be noninferior to combined modality treatment in interim-PET negative patients, the chemotherapy alone arm was discontinued.
Interim PET-directed ongoing studies in early-stage HL
| Reference . | Phase . | Sample . | Stage . | End point . | Pre-iPET CHT . | iPET− arm(s) . | iPET+ arm . | Group/title/NCT no. . | End year . |
|---|---|---|---|---|---|---|---|---|---|
| 41 | II | 660 | IA-IIAf | 5-y EFS | ABVD × 2 | INRT | ABVD × 2 ± INRTPET4− | RHC/NA | 2016 |
| IA-IIAuf | ABVD × 2 | ABVD × 2+INRT − | ABVD × 2 ± ABVD × 2 + INRTPET4 | 00392314 | |||||
| 42 | III | 1797 | IA-IIBf | PFS | ABVD × 2 | ABVD × 1 | escBEACOPP × 2 | EORTC-GELA/H10 | 2011 |
| IA-IIBuf | ABVD × 2 | escBEACOPP × 2 | 00433433 | ||||||
| 43 | II | 123 | IA-IIB blk | 3-y PFS | ABVD × 2 | ABVD × 4 | escBEACOPP × 4 | CALGB/50 801 | 2015 |
| + IFRT | 01118026 | ||||||||
| 44 | II | 200 | IA-IIB blk | 3-y PFS | ABVD × 2 | ABVD × 4 + INRT | escBEACOPP × 4* | ECOG/2410 | 2016 |
| + INRT | 01390584 | ||||||||
| 45 | II | 149 | IA-IIB n-blk | 3-y PFS | ABVD × 2 | ABVD × 2 | escBEACOPP × 2 | CALGB/50 604 | 2013 |
| + IFRT | 01132807 | ||||||||
| 46 | III | 575 | IA-IIA n-blk | PFS | ABVD × 3 | IFRT or | ABVD × 1 + IFRT | NHSFT/RAPID | 2012 |
| No therapy | 00943423 | ||||||||
| 47 | III | 1100 | I-IIAf | 5-y PFS | ABVD × 2 | No therapy | ABVD × 2 + IFRT | GHSG/HD16 | 2013 |
| 00736320 | |||||||||
| 48 | III | 1100 | I-IIrf | 3-y PFS | escBEACOPP × 2 + ABVD × 2 + IFRT | No therapy | escBEACOPP × 2 + ABVD × 2 | GHSG/ HD17 | 2013 |
| 01356680 |
| Reference . | Phase . | Sample . | Stage . | End point . | Pre-iPET CHT . | iPET− arm(s) . | iPET+ arm . | Group/title/NCT no. . | End year . |
|---|---|---|---|---|---|---|---|---|---|
| 41 | II | 660 | IA-IIAf | 5-y EFS | ABVD × 2 | INRT | ABVD × 2 ± INRTPET4− | RHC/NA | 2016 |
| IA-IIAuf | ABVD × 2 | ABVD × 2+INRT − | ABVD × 2 ± ABVD × 2 + INRTPET4 | 00392314 | |||||
| 42 | III | 1797 | IA-IIBf | PFS | ABVD × 2 | ABVD × 1 | escBEACOPP × 2 | EORTC-GELA/H10 | 2011 |
| IA-IIBuf | ABVD × 2 | escBEACOPP × 2 | 00433433 | ||||||
| 43 | II | 123 | IA-IIB blk | 3-y PFS | ABVD × 2 | ABVD × 4 | escBEACOPP × 4 | CALGB/50 801 | 2015 |
| + IFRT | 01118026 | ||||||||
| 44 | II | 200 | IA-IIB blk | 3-y PFS | ABVD × 2 | ABVD × 4 + INRT | escBEACOPP × 4* | ECOG/2410 | 2016 |
| + INRT | 01390584 | ||||||||
| 45 | II | 149 | IA-IIB n-blk | 3-y PFS | ABVD × 2 | ABVD × 2 | escBEACOPP × 2 | CALGB/50 604 | 2013 |
| + IFRT | 01132807 | ||||||||
| 46 | III | 575 | IA-IIA n-blk | PFS | ABVD × 3 | IFRT or | ABVD × 1 + IFRT | NHSFT/RAPID | 2012 |
| No therapy | 00943423 | ||||||||
| 47 | III | 1100 | I-IIAf | 5-y PFS | ABVD × 2 | No therapy | ABVD × 2 + IFRT | GHSG/HD16 | 2013 |
| 00736320 | |||||||||
| 48 | III | 1100 | I-IIrf | 3-y PFS | escBEACOPP × 2 + ABVD × 2 + IFRT | No therapy | escBEACOPP × 2 + ABVD × 2 | GHSG/ HD17 | 2013 |
| 01356680 |
iPET indicates interim PET; CHT, chemotherapy before interim PET imaging; esc, escalated; IPS, international prognostic score; INRT, involved-node radiotherapy; INRTPET4−, INRT is given to only those who have a PET-4 negative result (PET-4 positive arm will be salvaged); n-blk, nonbulky; blk, bulky; NA, not applicable; f, favorable; uf, unfavorable; rf, large mediastinal mass, extranodal involvement, elevated ESR, 3 or more involved nodal areas (stage IIB with risk factor 1 or 2 are not included); Rel/refrac, relapsed or refractory HL; GITIL, Gruppo Italiano Terapie Innovative Nei Linfomi, Italy; GELA-RC, Groupe d'Etude des Lymphomes de l'Adulte-Recherche Clinique, France; ECOG, Eastern Cooperative Oncology Group, United States; SWOG, Southwest Oncology Group, United States; CALGB, Cancer and Leukemia Group B (aka Alliance), United States; RHC, Rambam Health Care Campus, Israel; NCRI, Cancer Research, United Kingdom; NHSFT, NHS Foundation Trust, United Kingdom; GHSG, Deutsche Hodgkin Studiengruppe, Germany; and FIL, Fondazione Italiana Linfomi.
Therapy varies depending on the stage and risk group. Only PET− patients after BEACOPP × 4 will receive INRT; PET+ patients will be off study.
Extrapolating from prior FDG-PET data sets, a negative PET result after 2 cycles of ABVD is associated with 90%-95% progression-free survival (PFS) regardless of consolidative RT.6,50 It would be reasonable to use FDG-PET as a response surrogate after 2 cycles of ABVD with a plan to complete treatment with 2 more cycles of ABVD in the PET-negative group and complement therapy with 2 more ABVD courses followed by RT to those who have a PET-positive result. Several ongoing clinical trials are addressing this potential paradigm shift (H10, HD16, and RAPID; Table 1). Although the results of these trials will generate great interest, it is conceivable that the PFS will be slightly superior in the group receiving consolidative RT compared with those assigned to ABVD alone. Nonetheless, the clinicians should be cognizant of the fact that the objective is not merely to compare the treatment efficacy between the therapy arms but also to assess the benefits of omission of RT as a well-known risk factor for late toxicity. With the understanding that second-line treatments at the time of relapse can be quite effective in overcoming the apparent survival disadvantage, RT can be safely avoided. In line with this fact, the RAPID trial is powered around the proposition that a PFS that is 7% inferior is acceptable in the observation arm (PET-negative group) if RT can be avoided. The preliminary analysis of RAPID trial was reported on 571 patients, 74.6% of whom had a negative interim PET result.51 After a median follow up of 34.1 month, 389 of 420 (92.6%) were progression-free; 24 patients (5.7%) have progressed and 6 (1.4%) died yielding a combined 3-year PFS of 92.2% and overall survival (OS) of 98.2%.
In conclusion, the aforementioned PET-adapted treatments could, in the future, reduce the morbidity of the current chemoradiation-based therapeutic approach in early-stage HL, at least in the favorable-prognosis patient subgroup. However, adopting this strategy outside clinical trials should be strongly discouraged until the results of the ongoing trials are released with long-term follow-up data. Otherwise, increased risk of acute and late toxicities with no known outcome benefit, if any, cannot be justifiable without established grounds.
Interim response assessment: advanced-stage HL
The management objectives for advanced HL include both maximizing treatment efficacy and avoiding unnecessary toxicity for patients who do not require intensified treatment. Nevertheless, the primary treatment objective differs significantly from that of limited-stage HL, in that maximizing treatment efficacy takes precedence over minimizing therapy-related side effects. The pioneering studies by Hutchings and Gallamini have provided the proof of concept that early PET/CT imaging after 2 cycles of chemotherapy is a strong predictor of ultimate treatment outcome in patients with advanced-stage, ABVD-treated HL.7,8,13 In this category of patients, the issues related to a PET-adapted approach are double-fold. The first issue relates to the interim PET-negative group who is considered to have a high likelihood of a favorable outcome on the basis of excellent negative predictive value of PET imaging.7,8,13 However, this point may be confounded by high pretest probability for relapse, which might mitigate the predictive value (NPV) of PET. Nonetheless, this hypothetical scenario has been proven wrong, with a consistently high NPV of interim PET at ∼ 95%, despite the prediction of an adverse outcome by a high International Prognostic Score.13,52 There are at least 5 studies designed to test the survival equality with de-escalation of standard therapy in those patients with an interim PET negative result. These studies include the HD18 trial by the GHSG, investigating 6 cycles versus 4 cycles BEACOPP, the RATHL study comparing ABVD with AVD, the GELA AHL study comparing BEACOPP with standard ABVD, the HD 0607 and HD0801 trials by the Fondazione Italiana Linfomi investigating RT versus no RT after 6 cycles of ABVD in patients with interim and final PET-negative results (Table 2). The second thought-provoking issue is associated with the interim PET-positive group. Although the ramifications of a false-positive PET result would not affect this high-risk population as much as the limited-stage group, escalating therapy to a more toxic form would increase the toxicity and sterility risk of a fraction of patients who would otherwise be cured by conventional therapy.
Interim PET-directed ongoing studies in advanced-stage HL
| Reference . | Phase . | Sample . | Stage . | End point . | Pre-iPET CHT . | iPET− arm(s) . | iPET+ arm . | Group/title/NCT no. . | End year . |
|---|---|---|---|---|---|---|---|---|---|
| 53 | II | 450 | IIB-IV | 3-y PFS | ABVD × 2 | ABVD × 4 | escBEACOPP × 4 | GITIL/HD0607 | 2012 |
| stdBEACOPP × 4 | 00795613 | ||||||||
| 54 | II | 230 | III-IV | 2-y PFS | ABVD × 2 | ABVD × 2 | escBEACOPP × 6 | SWOG/S0816 | 2012 |
| 00822120 | |||||||||
| 55 | III | 798 | III-IV or IIB† | 5-y PFS | escBEACOPP × 2 | ABVD × 4 (experimental arm) | escBEACOPP × 4 (standard and experimental arms)‡ | GELA/AHL 2011 | 2016 |
| escBEACOPP × 4 (standard arm)‡ | 01358747 | ||||||||
| 56 | III | 1200 | IIβ-IV | 3-y PFS | ABVD × 2 | ABVD × 4 or AVD × 4 | escBEACOPP × 4 | CR-UK/RATHL | 2012 |
| 00678327 | |||||||||
| 57 | III | 1500 | IIB-IV | 5-y PFS | escBEACOPP × 2 | escBEACOPP × 6 or | escBEACOPP × 6 or | GHSG/HD18 | 2012 |
| escBEACOPP × 2 | escBEACOPP × 6 + rituximab | 00515554 | |||||||
| 58 | III | 300 | IIB-IV | 2-y PFS | ABVD × 2 | ABVD × 4Ω | IGEV × 4 + ASCT | FIL/ HD0801 | 2014 |
| 00784537 | |||||||||
| 59 | II | 660* | III-IV0-2 | 5-y EFS | ABVD × 2 | ABVD × 4 | escBEACOPP × 2 + 00392314 | RHC/NA | 2016 |
| escBEACOPP × 2 + INRTPET4− | |||||||||
| III-IV3-7 | escBEACOPP × 2 | ABVD × 4 | escBEACOPP × 2 + | ||||||
| escBEACOPP × 2 + INRTPET4− |
| Reference . | Phase . | Sample . | Stage . | End point . | Pre-iPET CHT . | iPET− arm(s) . | iPET+ arm . | Group/title/NCT no. . | End year . |
|---|---|---|---|---|---|---|---|---|---|
| 53 | II | 450 | IIB-IV | 3-y PFS | ABVD × 2 | ABVD × 4 | escBEACOPP × 4 | GITIL/HD0607 | 2012 |
| stdBEACOPP × 4 | 00795613 | ||||||||
| 54 | II | 230 | III-IV | 2-y PFS | ABVD × 2 | ABVD × 2 | escBEACOPP × 6 | SWOG/S0816 | 2012 |
| 00822120 | |||||||||
| 55 | III | 798 | III-IV or IIB† | 5-y PFS | escBEACOPP × 2 | ABVD × 4 (experimental arm) | escBEACOPP × 4 (standard and experimental arms)‡ | GELA/AHL 2011 | 2016 |
| escBEACOPP × 4 (standard arm)‡ | 01358747 | ||||||||
| 56 | III | 1200 | IIβ-IV | 3-y PFS | ABVD × 2 | ABVD × 4 or AVD × 4 | escBEACOPP × 4 | CR-UK/RATHL | 2012 |
| 00678327 | |||||||||
| 57 | III | 1500 | IIB-IV | 5-y PFS | escBEACOPP × 2 | escBEACOPP × 6 or | escBEACOPP × 6 or | GHSG/HD18 | 2012 |
| escBEACOPP × 2 | escBEACOPP × 6 + rituximab | 00515554 | |||||||
| 58 | III | 300 | IIB-IV | 2-y PFS | ABVD × 2 | ABVD × 4Ω | IGEV × 4 + ASCT | FIL/ HD0801 | 2014 |
| 00784537 | |||||||||
| 59 | II | 660* | III-IV0-2 | 5-y EFS | ABVD × 2 | ABVD × 4 | escBEACOPP × 2 + 00392314 | RHC/NA | 2016 |
| escBEACOPP × 2 + INRTPET4− | |||||||||
| III-IV3-7 | escBEACOPP × 2 | ABVD × 4 | escBEACOPP × 2 + | ||||||
| escBEACOPP × 2 + INRTPET4− |
std indicates standard. See Table 1 for other abbreviations.
This study has 4 arms involving both early- and advanced-stage HL (thus mentioned in Table 1 as well); 0-2, patients with IPS 0-2; 3-4, patients with IPS 3-7.
High-risk IIB stage, β, stage IIB, or IIA disease with adverse features, Ω, PET2-negative patients randomized to IFRT versus no IFRT based on initial bulky disease.
For patients with a positive PET-4 (interim PET scan after 4 cycles of chemotherapy) a rescue treatment is followed with HDT/ASCT.
Several trials were designed to initiate first-line therapy with the most intensified regimen (BEACOPPescalated) and subsequently de-escalate treatment in patients with a negative interim PET to overcome chemoresistance early during treatment. This approach is currently being tested in the GHSG HD-18 clinical trial (Table 2). The interim analysis of this trial was reported in 240 patients.60 Using modified Deauville criteria with a highly sensitive threshold for interim PET scan positivity, 98 patients (41%) were PET-2 positive and 142 (59%) PET-2 negative; these numbers are updated in Table 3 after personal communication with the primary investigators. There are 2 other ongoing trials at the time of writing of this article, by the Group pour l'Etude des Lymphomes de l'Adulte (GELA) and the national Israel lymphoma study group, both starting with BEACOPPescalated regimen and randomizing PET-2 negative patients to continue on BEACOPPescalated or de-escalate treatment to ABVD in the experimental arm. The results of interim analyses of these trials are still pending (Table 2). The alternative strategy would be to start with “standard” ABVD treatment and escalate to BEACOPPescalated only in a small patient subset (nearly 20%) who has a positive interim PET. This approach is the principal end point of several prospective international studies following a similar strategy (Table 2). In the first interim analysis of the Italian HD 0607 study, 13 of 17 PET-2 positive patients (76%) became PET-negative at the end of treatment and continued to have a durable CR.61
Current status of interim PET-directed ongoing trials in HL
| Group/study . | Reference . | Stage . | Recruited/target . | iPET+ . | iPET− . | Interpretation rules . |
|---|---|---|---|---|---|---|
| GITIL/HD0607 | 53 | Advanced | 389/450 | 77 (20%) | 312 (80%) | 5PS |
| SWOG/ S0816 | 54 | Advanced | 248/300 | 45 (20%) | 176 (80%) | 5PS |
| GELA/AHL 2011 | 55 | Advanced | 100/798 | — | — | 5PS |
| CR-UK/RATHL | 56 | Advanced | 680/1200 | 106 (16%) | 574 (84%) | 5PS |
| GHSG/HD18 | 57 | Advanced | 1146/1500 | 535 (47%) | 611 (53%) | Modified 5PS |
| FIL/ HD0801 | 58 | Advanced | 291/300 | 70 (24%) | 221 (76%) | IHP |
| RHC/NA | 59 | Early + advanced | 226/660 | 29 (13%) | 197 (87%) | Dynamic |
| EORTC-GELA/H10 | 42 | Early | 124/1797 | 24 (19%) | 100 (81%) | Modified IHP |
| CALGB/50801 | 43 | Bulky early | 17/123 | 4 (31%) | 9 (69%) | 5PS |
| ECOG/2410 | 44 | Bulky early | —/200 | — | — | 5PS |
| CALGB/50604 | 45 | n-bulky early | 75/149 | 5 (9%) | 51 (91%) | 5PS |
| NHS-FT/RAPID | 46 | n-bulky early | 571/575 | 145 (25%) | 426 (75%) | 5PS |
| GHSG/HD16 | 47 | f-Early | —/1100 | — | — | Modified 5PS |
| GHSG/HD17 | 48 | Early rf | —/1100 | — | — | Modified 5PS |
| Group/study . | Reference . | Stage . | Recruited/target . | iPET+ . | iPET− . | Interpretation rules . |
|---|---|---|---|---|---|---|
| GITIL/HD0607 | 53 | Advanced | 389/450 | 77 (20%) | 312 (80%) | 5PS |
| SWOG/ S0816 | 54 | Advanced | 248/300 | 45 (20%) | 176 (80%) | 5PS |
| GELA/AHL 2011 | 55 | Advanced | 100/798 | — | — | 5PS |
| CR-UK/RATHL | 56 | Advanced | 680/1200 | 106 (16%) | 574 (84%) | 5PS |
| GHSG/HD18 | 57 | Advanced | 1146/1500 | 535 (47%) | 611 (53%) | Modified 5PS |
| FIL/ HD0801 | 58 | Advanced | 291/300 | 70 (24%) | 221 (76%) | IHP |
| RHC/NA | 59 | Early + advanced | 226/660 | 29 (13%) | 197 (87%) | Dynamic |
| EORTC-GELA/H10 | 42 | Early | 124/1797 | 24 (19%) | 100 (81%) | Modified IHP |
| CALGB/50801 | 43 | Bulky early | 17/123 | 4 (31%) | 9 (69%) | 5PS |
| ECOG/2410 | 44 | Bulky early | —/200 | — | — | 5PS |
| CALGB/50604 | 45 | n-bulky early | 75/149 | 5 (9%) | 51 (91%) | 5PS |
| NHS-FT/RAPID | 46 | n-bulky early | 571/575 | 145 (25%) | 426 (75%) | 5PS |
| GHSG/HD16 | 47 | f-Early | —/1100 | — | — | Modified 5PS |
| GHSG/HD17 | 48 | Early rf | —/1100 | — | — | Modified 5PS |
— indicates not applicable; 5PS, 5-point scale (Deauville criteria); Modified 5PS, Modified 5-point scale; IHP, International Harmonization Project criteria for end-treatment PET evaluation72 ; Dynamic, dynamic interpretation criteria developed by the Israeli group for interim PET evaluation73 ; and Modified IHP, modified International Harmonization Project criteria for end-therapy PET evaluation.
Interim PET outside clinical trials: problems and interpretation criteria
In the absence of published prospective multicentre data, it is unclear whether a PET-adapted strategy could revolutionize management by providing an individualized “metabolic fingerprint,” with beneficial consequences of altered treatment algorithms in HL patients.62,63
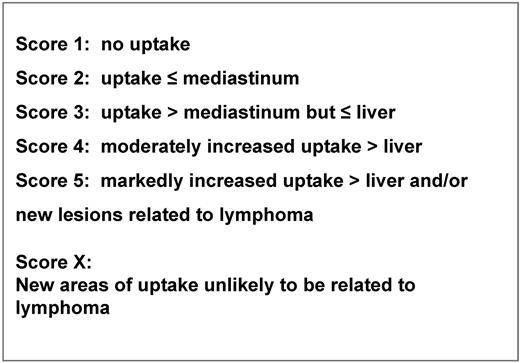
Limited and preliminary reports from a retrospective study64 and a prospective trial,65 have demonstrated an apparent advantage with respect to failure-free survival using a PET-adapted strategy as compared with the conventional treatment schedule. However, no direct randomized comparison is currently available comparing a PET response-adapted strategy versus the standard chemotherapy regimen without any alteration during treatment. Consequently, interim PET should be considered an investigational procedure; thus, planned therapy schemes should not be changed based on interim PET results outside well-designed clinical trials.66 Ultimately, the results of ongoing clinical trials will provide guidance regarding the predictive and prognostic value of interim PET and survival advantage for PET response-adapted strategy. Nonetheless, the practice of PET scanning early during treatment has become quite pervasive and consequently, at some centers, an interim PET scan is now prematurely used as a decision tool integrated in the therapeutic algorithms. However, the absence of standardized universal reporting criteria hampers the effective use of interim PET scans during routine HL treatment practices. In 2009, at a meeting held in Deauville, a panel of oncologists and imaging experts came to an agreement that qualitative determination of residual FDG uptake was appropriate using a reference anatomic site such as the mediastinal blood pool or the liver for grading the FDG uptake.67 Because therapy response cannot ideally be assessed using dichotomous criteria but rather using a continuous response scheme, after consecutive international workshops, a consensus was reached to propose a graded 5-point scale to visually assess FDG uptake to accommodate the flexibility needed by the clinicians68,69 (Figure 1). Similar interpretation criteria proved quite reliable and reproducible with a favorable concordance rate among reviewers in the RATHL (Risk-adapted therapy for Hodgkin Lymphoma) and the IVS (International validation study for interim-PET in Hodgkin Lymphoma) studies.70,71 Naturally, the cut-off definition for a positive and a negative PET result in a scale of increasing FDG uptake intensity is impacted by the intent of the planned treatment (ie, intensification or de-intensification), which would allow for a more permissive or restrictive reading. A high positive predictive value and specificity and a high sensitivity and NPV can be a trade-off for therapy intensification and de-intensification, respectively, to avoid over- and under-treatment. Thus, a graded method of visual criteria that is conducive to different positivity thresholds could be used to adjust for the required test specificity in a given trial design.70 Nonetheless, other interpretation schemes have been proposed for interim PET results based on various assumptions, using the same set of criteria as those used for end-therapy PET,72 or increasing the threshold for a positive scan to improve specificity,70,71 or comparing the results of baseline versus interim PET scan using a dynamic score73 (Table 3).
For interim PET interpretation in HL, the visual assessment, as recommended by the experts, is currently preferred over the semiquantitative evaluation using standardized uptake values. Indeed, the level of residual activity varies during treatment with the kinetics of tumor cell kill, histologic subtype of lymphoma, tumor microenvironment, treatment intensity, and the time point at which PET is performed (after 2 cycles or more). The characteristic cell composition of HL, the high chemosensitivity of this neoplasm, and the abrupt shut-off of chemokine production of the bystander cells with cytotoxic effects of chemotherapy account for the rapidity of metabolic complete response, which is achieved with a “on-off” pattern.18-20 Quantitative evaluation using standardized uptake value measurements may be more suitable for aggressive B-cell lymphomas where the residual FDG uptake results from a balanced combination between cell kill and tumor regrowth.74
There is no ideal gold standard to test the validity of interim PET scan results. As previously alluded, not only does the tissue biopsy have significant limitations,75 but also histologic confirmation could not be advocated as a reference test for interim-PET scan because the latter was proposed as a surrogate of tumor chemosensitivity and not to assess an early complete response to chemotherapy. In an attempt to validate the proposed Deauville 5-point scoring (5PS) system, the retrospective international validation study (IVS) was undertaken to test the interim-PET results with a primary end point of 3-year failure-free survival. In a cohort of 260 advanced-stage HL patients imaged after 2 cycles of ABVD, without any treatment change based on PET-2 results, 45 patients were PET-2 positive and 215 were PET-2 negative.52 After a mean follow-up of 27.2 months, the 3-year PFS of PET-2 positive and negative patients were 28% and 95%, respectively (P < .001). The binary concordance between paired reviewers for positive versus negative results was very good (κ Cohen = 0.84).
Interim PET to guide postchemotherapy consolidation radiotherapy
As alluded before (see “Introduction”), 60%-80% of HL patients will have a residual mass at completion of chemotherapy, the majority of which is confined to sites of previous bulky disease.23,24 However, only < 50% of these masses will harbor residual disease.14 In advanced-stage HL patients, ABVD followed by IFRT for bulky nodal lesions or residual masses was proposed to consolidate chemotherapy response.3 More recently, end-of-therapy PET/CT proved effective in discrimination between residual active disease and fibrotic mass, with a sensitivity of 0.43-1.00 for and specificity of 0.67-1.00.22 These results were highly encouraging with the caveat that residual FDG uptake does not always represent presence of active disease because of its inability to discriminate between viable tumor and florid inflammatory response.76 Preliminary studies suggested that, in neoplasms, the FDG accumulation continues to increase over time in malignant lesions while decreasing in infectious or inflammatory processes. This difference in the time course of FDG accumulation, so-called “dual-point imaging,” with imaging at 2 different time points could be useful to improve the specificity of FDG-PET imaging in the residual masses.77 The pilot data demonstrated promising results in HL; however, further investigation is warranted to confirm these results.40 In the HD15 trial of the GHSG, consolidation radiotherapy was selectively administered to advanced-stage HL patients who had a PET-positive residual mass of > 2.5 cm at the end of chemotherapy, using 3 different BEACOPP regimens. The 4-year PFS of irradiated and nonirradiated patients were 86.2% and 92.6%, respectively (P = .022). The NPV of end-therapy PET was 94%.26 These data suggest that radiotherapy can be safely omitted in advanced-stage HL patients who are PET-negative after BEACOPPescalated treatment. It should be emphasized, however, that the NPV of end-therapy PET depends on the effectiveness of chemotherapy administered; indeed, a lower NPV (86%) was reported with less effective regimens.78 The obvious question of whether or not the results of the HD15 trial also apply to patients treated with ABVD and to those with initial bulky disease was addressed by the British Columbia Cancer Agency in a retrospective analysis. In 163 advanced-stage HL patients with a residual mass of ≥ 2 cm after ABVD therapy, those patients who had a FDG-avid mass were treated with consolidation RT and the others were observed.79 Patients with a PET-negative scan (n = 130, 80%) had a far superior 3-year time to progression compared with those with a PET-positive scan (89% vs 55%, P = .00001) with no difference between those with bulky versus nonbulky disease. These results strongly support the omission of RT in advanced stage HL patients who achieve a PET-negative remission after 6 cycles of chemotherapy, whereas the decision to irradiate a PET-positive lesion should be made with the awareness of false-positive results as well as the lack of success in those with radiation-resistant disease.
Interim PET during rescue treatment for relapsed/refractory HL
Despite a high cure rate, the standard ABVD treatment with or without RT fails to control disease in ∼ 25% of HL patients, either on the basis of resistant or relapsing lymphoma.2,3,66 The current standard second-line treatment for relapsed or refractory HL involves high-dose chemotherapy and autologous hematopoietic stem cell transplantation (HDT/ASCT), producing long-term disease free survivals in up to 65% of patients.80,81 Favorable outcome depends largely on chemosensitivity at the time of ASCT. Despite the heterogeneity of included patient groups and the different number of previous chemotherapy lines across various studies, meta-analysis data confirmed the prognostic impact of pretransplant FDG-PET in lymphoma patients, providing a uniform measure of the association for both PFS and survival after ASCT.82 Pooled survival data suggested a worse PFS and OS after a positive FDG-PET study (HR = 3.2, and 4.5, respectively). More recent studies reported on uniform HL patient populations confirmed the powerful predictive value of pretransplant FDG-PET imaging on the success of ASCT.83,84 Moskowitz et al reported on the outcome of patients with relapsed HL who had chemosensitive disease after ifosfamide, carboplatin, and etoposide (ICE) salvage therapy and were undergoing ASCT: 153 patients with chemosensitive disease after ICE-based salvage therapy proceeded to high-dose chemoradiotherapy followed by ASCT. Functional imaging status using gallium scan or FDG-PET before ASCT was the only factor significant for event-free survival (EFS) and OS by multivariate analysis and identified poor-risk patients (5-year EFS 31% and 75% for imaging-positive and -negative patients, respectively).
More recently, in a phase 2 study, using both presalvage therapy prognostic factors and post-salvage PET response in a risk-adapted approach to improve PFS after HDT and ASCT, Moskowitz et al demonstrated that patients transplanted with a negative PET result before, had an EFS of > 80%, versus 28.6% for patients with a positive PET result (P < .001).84 This prospective study provided further evidence that the primary objective of salvage therapy in patients with HL should be achieving a negative FDG-PET result before pursuing HDT/ASCT.
The literature on the prognostic role of interim PET during second-line salvage is insufficient than during induction therapy but equally in favor of the prognostic role of PET scan. In a small cohort of 24 patients treated with second-line chemotherapy with ifosfamide, gemcytabine, and vinorelbine, followed by ASCT for relapsing/refractory HL, PET scan performed after the second cycle was predictive of treatment outcome. The 2-year PFS was 93% versus 10% for patients with negative and positive interim PET, respectively (P < .001).85 Even less is known on the role of interim PET in relapsed/refractory HL patients treated with brentuximab-vedotin (BV). However, given that the median time to objective response to BV treatment is ∼ 6 weeks in refractory/relapsed HL patients,86 performing an interim PET after 2 or 3 courses of BV is considered reasonable as evidenced in several trials.87
PET scan timing and protocol standardization
Interim PET should not be performed 7-10 days after chemotherapy infusion to avoid the critical window of inflammatory response peaking during this period.72,76 Imaging at an earlier time period increases the risk of compromise test sensitivity because of stunning of cellular glucose metabolism by immediate effect of chemotherapy.88 Therefore, the recommended timing for interim PET scan is day 11-13 of the second chemotherapy administration, considering that two 14-day treatments account for one cycle in the HL treatment schedule. The timing of post-therapy completion FDG-PET studies should be performed 3-6 weeks after completion of the last cycle.72 However, if RT is involved, 12-week interval is preferred to minimize false-positive results related to RT induced inflammation.88
The importance of adherence to international PET guidelines cannot be emphasized enough. Standardized image acquisition guidelines should be followed for consistently reliable data and for intra- as well as inter-institutional cross-comparative studies.89,90 In the aforementioned IVS study, only 39% of patients were found to undergo a PET scan performed in accordance with the aforementioned guidelines.52 Positive predictive value of interim PET in predicting treatment outcome in the entire patient cohort and in the patient subset scanned according international guidelines was 0.73 and 0.86, respectively (P < .01; Gallamini et al91 ). These data suggest that although integrated PET/CT has been widely adopted, protocol standardization has not kept up pace with the expansion in PET applications.
In conclusion, the current paradigm in HL management is to achieve a balance between maximizing the likelihood of cure and minimizing therapy-related late side effects. In this conjecture, interim PET imaging has been proposed as a useful prognostic tool integrated in a response-adapted therapy setting. A multitude of trials are currently underway to test the accuracy of PET as a marker of tumor chemosensitivity. However, whether a PET-adapted individualized treatment strategy leads to a long-term survival benefit compared with standard chemotherapy remains unknown for the HL population. Hence, in clinical practice, HL therapy should be individualized based on established clinical factors but not on interim PET until the ongoing trials provide more definitive guidance about the impact of interim PET-tailored approach.
Acknowledgments
The authors thank Anna Cavallo (Hematology Department, Cuneo) for data editing and text formatting.
Authorship
Contribution: A.G. and L.K. contributed to the article in their respective fields of expertise (A.G. as a hemato-oncology expert in HL clinical management and integration of functional imaging in response assessment and L.K. is a nuclear medicine doctor with prevalent experience in imaging and imaging reporting–related problems in lymphoma) and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea Gallamini, Hematology Department and BMT Unit, Azienda Ospedaliera S. Croce e Carle, Via M. Coppino 26, 12100 Cuneo, Italy; e-mail: gallamini.a@ospedale.cuneo.it.