Abstract
In cancer, VEGF-induced increase in vascular permeability results in increased interstitial pressure, reducing perfusion and increasing hypoxia, which reduce delivery of chemotherapeutic agents and increase resistance to ionizing radiation. Here, we show that both TIMP-2 and Ala + TIMP-2, a TIMP-2 mutant without matrix metalloproteinase inhibitory activity, antagonize the VEGF-A–induced increase in vascular permeability, both in vitro and in vivo. Like other agents known to preserve endothelial barrier function, TIMP-2 elevates cytosolic levels of cAMP and increases cytoskeletal-associated vascular endothelial cadherin in human microvascular endothelial cells. All of these effects are completely ablated by selective knockdown of integrin α3β1 expression, expression of a dominant negative protein tyrosine phosphatase Shp-1 mutant, administration of the protein tyrosine phosphatase inhibitor orthovanadate, or the adenylate cyclase inhibitor SQ22536. This TIMP-2–mediated inhibition of vascular permeability involves an integrin α3β1-Shp-1-cAMP/protein kinase A-dependent vascular endothelial cadherin cytoskeletal association, as evidenced by using siRNAs to integrin α3β1 and Shp-1, or treatment with Shp-1 inhibitor NSC87877 and protein kinase A inhibitor H89. Our results demonstrate the potential utility for TIMP-2 in cancer therapy through “normalization” of vascular permeability in addition to previously described antiangiogenic effects.
Introduction
Tumor-associated angiogenesis is critical for tumor progression and metastasis. The central role of vascular endothelial growth factor-A (VEGF-A) in this process is evidenced by the development and approval of bevacizumab, a VEGF-A neutralizing antibody, for therapy in several human cancers. VEGF-A–induced angiogenesis is often accompanied by increased vascular permeability, which can result in fibrin deposition and may facilitate tumor cell extravasation enhancing metastasis formation.1 The resulting vascular leak has also been shown to increase interstitial pressure within the tumor, decrease tumor blood flow, and hinder drug delivery to the tumor. Indeed, it has been proposed that VEGF-axis targeted therapies may result in “normalization” of tumor vasculature improving chemotherapeutic delivery and decreasing hypoxia, resulting in enhanced radiosensitivity.2,3
Vascular permeability can be modulated by the phosphorylation, cleavage, and internalization of vascular endothelial (VE)–cadherin.4-6 Tyrosine phosphorylation of the cadherin-catenin complexes is regulated by the activities of protein tyrosine phosphatases and src-family kinases.7-11 Inhibition of tyrosine phosphorylation of VE-cadherin increases the stability of adherens junctions and improves vascular barrier function. Matrix metalloproteinase (MMP)–mediated cleavage of VE-cadherin may promote vascular permeability and cell proliferation by dissociating cadherin-catenin complex and disrupting cell-cell adhesion.12-15 In contrast, it is widely recognized that, in endothelial cells, an increase in cAMP enhances barrier function and reduces vascular permeability in vitro and in vivo.16-18
The mammalian tissue inhibitor of metalloproteinase (TIMP) family consists of 4 members (TIMP-1, -2, -3, and -4), which share significant homology and bind to the catalytic site of activated MMPs, leading to inhibition of protease activity.19-21 TIMPs may also bind to the carboxyl-terminal hemopexin-like domain of specific proforms of MMP family members and regulate their cell surface activation.20-22 In addition, TIMPs have pluripotential effects on cell growth, migration, and differentiation.23-28 TIMP-2 is unique in that it is the only member of the TIMP family that has been shown to inhibit angiogenesis independent of MMP inhibitory activity.24,25 Fernandez et al reported that the carboxyl-terminal domain of TIMP-2, in particular Loop 6, inhibits endothelial cell proliferation and angiogenesis.25 These antiangiogenic effects of Loop 6 are mediated by direct binding to insulin-like growth factor receptor I (IGF-IR) and subsequent regulation of IGF-IR signaling pathways.25,29
TIMP-2 binds to the surface of human microvascular endothelial cells (hMVECs) via interaction with the integrin α3β1, and this interaction suppresses fibroblast growth factor-2 (FGF-2)– or VEGF-A–induced endothelial cell proliferation in vitro, angiogenesis, and tumorigenesis in vivo.24,30-33 These TIMP-2 antiangiogenic effects are mediated by SH2-containing protein tyrosine phosphatase-1 (Shp-1)–dependent suppression of receptor tyrosine kinase (RTK) signaling pathways and induction of cyclin-dependent kinase (cdk) inhibitor p27Kip1, resulting in hypophosphorylation of Rb and cell cycle arrest.30,31 We have also demonstrated that TIMP-2 inhibits endothelial cell migration through inhibition of src, inhibition of paxillin phosphorylation, and activation of Rap1.26,34
Given the effects of TIMP-2 on VEGF-induced angiogenesis described earlier in the “Introduction,” we examined the effects of exogenous TIMP-2 on endothelial barrier function. One of the earliest effects of VEGF-A on endothelial cells is the dissociation of adherens junctions and redistribution of VE-cadherin into a detergent (Triton X-100) soluble compartment.35,36 In the present study, we report that TIMP-2 suppresses VEGF-A–induced vascular permeability by enhancing VE-cadherin association with the cytoskeleton as demonstrated by an increase in expression of the adherens junctions (a decrease in Triton X-100 solubility). These effects are dependent on Shp-1, adenylate cyclase (AC), and protein kinase A (PKA) activity.
Methods
Reagents
Recombinant vascular endothelial growth factor-A was purchased from Millipore. The following pharmacologic agents and antibodies were obtained from commercial sources: β-adrenergic receptor agonist isoproterenol, AC inhibitor SQ22536, AC activator forskolin, and PKA inhibitor H89 (Sigma-Aldrich); PKA activator N6-benzoyl-cAMP (6-Bnz-cAMP) and exchange factors directly activated by cAMP (Epac) activator 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-Me-cAMP; Biolog Life Science Institute); protein tyrosine phosphatase inhibitor 8-hydroxy-7-[(6-sulfo-2-naphthyl)azo]-5-quinolinesulfonic acid (NSC87877) and MMP inhibitor BB94 (Tocris Bioscience); anti–VE-cadherin (Chemicon International); anti-phosphotyrosine (PY20), anti–Shp-1, anti-integrin α5, anti-integrin β1, anti-integrin β3, anti–α-catenin, anti–β-catenin, anti–γ-catenin, and anti–p120-catenin (BD Biosciences); anti-integrin α3, anti–VEGFR-2, anti–VE-cadherin, antiactin, and mouse IgG-horseradish peroxidase conjugate (Santa Cruz Biotechnology). TIMP-2 and Ala + TIMP-2 were prepared and characterized as described previously.37
Cell culture conditions
hMVECs were purchased from Lonza Walkersville and used as previously described.24 Human lung carcinoma cells (A549) from ATCC were grown in 10% FBS-DMEM (Invitrogen). GD25 β1 null murine embryonic fibroblasts and GD25-β1A cells were kindly provided from Dr Deane F. Mosher (University of Wisconsin, Madison, WI).
cAMP determination
hMVECs in gelatin-coated 100-mm dishes (BD Biosciences) were serum-starved for 18 hours, followed by the indicated period of incubation with TIMP-2 (100nM) at 37°C; the cells were washed once with PBS (pH 7.4) and twice more with 50mM Tris-HCl (pH 7.5) containing 4mM EDTA and 1mM 3-isobutyl-1-methylxanthine. Cell lysates were prepared with lysis buffer containing 50mM Tris-HCl (pH 7.5), 4mM EDTA, 1mM 3-isobutyl-1-methylxanthine, 150mM NaCl, and 1% Nonidet P-40 for 20 minutes at 4°C, followed by centrifugation at 13 000g for 20 minutes at 4°C, and by heating for 5 minutes in a boiling water bath to coagulate protein. After centrifugation the cAMP levels in the supernatants were determined by [3H]cAMP assay system (GE Healthcare) according to the manufacturer's instructions, normalized to the protein concentration, and expressed as femtomoles of cAMP per microgram of protein or the fold increase of nonstimulated hMVECs to allow comparison between experiments.
RNA interference
Both the shRNA-targeting integrin α3 gene and scrambled sequences cloned into psiRNA-hH1zeo vector (InvivoGen) were designated α3 shRNA and control shRNA, respectively. These vector constructs were transfected into A549 lung carcinoma cells using LyoVec (InvivoGen) as previously described.30 For transfection of hMVECs, the integrin α3, Shp-1, and control siRNA duplexes were purchased from Santa Cruz Biotechnology. hMVECs in gelatin-coated 100-mm dishes were transfected with siRNA (integrin α3 siRNA (sc-35684), 50nM; Shp-1 siRNA (sc-29478), 50nM; Shp-2 siRNA (sc-36488), 50nM; control siRNA (sc-37007), 50nM) using Lipofectamine RNAiMAX (Invitrogen) and Opti-MEM I reduced serum media (Invitrogen). The concentrations of siRNAs were chosen based on dose-response studies.
Dominant negative Shp-1 adenovirus
Expression and loss of activity of dominant negative (dn) Shp-1 in adenovirus infected cells were verified by Western blotting using anti–Shp-1 antibody (BD Biosciences) and protein tyrosine phosphatase assay (Upstate Biotechnology) of whole cell lysates, respectively, as previously described.24
Immunofluorescence microscopy
hMVECs on gelatin- or laminin-coated coverslips in 24-well plates were serum-starved for 18 hours, pretreated with or without TIMP-2 or Ala + TIMP-2 for 15 minutes, fixed with 3.7% paraformaldehyde for 5 minutes, washed with PBS, permeabilized with 0.1% Triton X-100 for 10 minutes, washed with PBS, and blocked with PBS containing 5% BSA for 30 minutes. Primary antibodies diluted 1:100 in 5% BSA-PBS were incubated for 2 hours at room temperature, washed with PBS, and followed by Alexa Fluor-488–conjugated goat anti–mouse IgG or Alexa Fluor-546–conjugated goat anti–rabbit IgG (Invitrogen). The F-actin cytoskeleton was stained with phalloidin-TRITC (Sigma-Aldrich) diluted 1:200 in PBS for 1 hour at room temperature. Images were obtained with an Olympus FV300 confocal laser scanning microscope (FV300 and IX-81, UPlanSApo 40×/0.95 objective) and Olympus Fluoview Version 1.7b software (Olympus).
Permeability assay
Permeability across the endothelial cell monolayer was measured using fluorescence blocking PET track-etched membrane 24-well format units (HTS FluoroBlok Insert, 3.0-μm pore size; BD Biosciences). hMVECs, plated on collagen- or laminin-coated transwell inserts (6 × 104 cells/well) in 24-well plates, were grown in EGM-2 MV BulletKit media for 2-3 days until they had reached confluence. After starvation in basal EBM-2 media without serum and growth factors for 1 hour, FITC-dextran (final concentration of 1 mg/mL, molecular weight, 40 kDa; Sigma-Aldrich) was added to the upper chamber, and the cells were immediately treated with TIMP-2, cAMP, forskolin, BB94, or VEGF-A as indicated in the figure legends. The fluorescence was measured at 490/520 nm with PerkinElmer HTS7000 fluorometer (PerkinElmer Life and Analytical Sciences).
In vivo permeability assay
Quantification of in vivo permeability was performed using the modified Miles assay as described previously.38 ICR mice received a 100-μL tail vein injection of 1% Evans blue dye in PBS followed by shaving a patch of dorsal skin. The mice then received intradermal injections with 50 μL of VEGF-A (1 μg/mL) alone or with TIMP-2 (500nM), or Ala + TIMP-2. After 30 minutes, the mice were killed, the intradermal injection sites were photographed, excised, and extravasated blue dye was eluted with formamide at 56°C and measured by spectrometry at 620 nm. The animal studies were performed in accordance with the institutional guidelines. The experimental protocol had received prior approval from the Institutional Animal Care and Use Committee at Kangwon National University.
Histology
Tissues were fixed with 3.7% paraformaldehyde and processed using routine paraffin-embedding procedures. Sections (4-μm thickness) were stained with hematoxylin and eosin before microscopic examination.
Immunoprecipitation and Western blot analysis
Quiescent hMVECs were replaced with fresh basal EBM-2, followed by treatments for the indicated time points at 37°C. hMVECs were rinsed twice with ice-cold PBS and lysed by incubation in 50mM Tris-HCl, pH 7.4, 150mM NaCl, 10% glycerol, 1% Triton X-100, 1mM EDTA, 100 μg/mL 4-(2-aminoethyl)benzenesulfonyl fluoride, 10 μg/mL aprotinin, 1 μg/mL pepstatin A, 0.5 μg/mL leupeptin, 80mM β-glycerophosphate, 25mM NaF, and 1mM sodium orthovanadate for 30 minutes at 4°C. Cell lysates were clarified at 13 000g for 20 minutes at 4°C, and the supernatants were subjected to immunoprecipitation and Western blot as described previously.24 All immunoprecipitations and Western blots were performed at least triplicate experiments, and representative gels are shown. Bands of interest were integrated and quantified by the use of National Institutes of Health (NIH) ImageJ Version 1.34s software.
Zymogram analysis
Activities of MMP-2 were measured by zymography.39 Aliquots of conditioned medium were diluted in sample buffer, applied to 10% polyacrylamide gels containing 1 mg/mL gelatin (Sigma-Aldrich) as a substrate. After electrophoresis, the gels were incubated in 2.5% Triton X-100 for 1 hour to remove SDS and allow renaturalization of MMPs, and further incubated in developing buffer containing 50mM Tris (pH 7.5), 10mM CaCl2, and 150mM NaCl for 20 hours at 37°C. The gels were stained with 0.5% Coomassie brilliant blue R-250 in 30% methanol-10% acetic acid for 2 hours and followed by destaining with 30% methanol-10% acetic acid. Gelatinolytic activities were detected as unstained bands against the background of the Coomassie blue-stained gelatin.
Statistical analysis
Statistical analysis was performed using Student t test and was based on at least 3 different experiments. The results were considered to be statistically significant when P < .05.
Results
TIMP-2 antagonizes the VEGF-A–induced increase in vascular permeability through Shp-1 activity
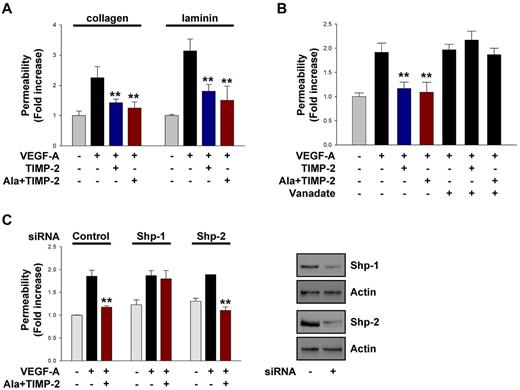
Treatment with TIMP-2 or Ala + TIMP-2 (100nM) markedly abrogated the VEGF-A–induced (100 ng/mL) increase in endothelial permeability in hMVECs on collagen and laminin (Figure 1A). The role of protein tyrosine phosphatase (PTP) activity in TIMP-2 inhibition of VEGF-A–stimulated endothelial permeability was examined by the use of the PTP inhibitor orthovanadate on hMVEC monolayers. As shown in Figure 1B, pretreatment with orthovanadate did not significantly alter the VEGF-A–induced increase in endothelial permeability but prevented the TIMP-2–mediated decrease in VEGF-stimulated endothelial permeability. These results demonstrate that TIMP-2 reduces VEGF-A–induced increase in vascular permeability in an MMP-independent manner that requires PTP activity.
TIMP-2 inhibits VEGF-A–induced endothelial cell permeability through PTP Shp-1 activity. (A) Quiescent hMVECs were pretreated with TIMP-2 (100nM) or Ala + TIMP-2 for 20 minutes and followed by VEGF-A (100 ng/mL) stimulation for 30 minutes. The results (mean ± SD) are expressed as the fold increase of FITC-dextran permeability in untreated control cells. **P < .01, compared with VEGF-A treatment alone. (B) Cells were pretreated with orthovanadate (2μM) for 20 minutes and followed by TIMP-2 or Ala + TIMP-2 before VEGF-A stimulation for 30 minutes. **P < .01, compared with VEGF-A treatment alone. (C) Cells transfected with control, Shp-1, or Shp-2 siRNA were treated as in panel A. **P < .01, compared with VEGF-A treatment alone. Cell lysates were Western blotted with anti-Shp-1, anti-Shp-2, or antiactin antibodies. Results are representative of 3 independent experiments.
TIMP-2 inhibits VEGF-A–induced endothelial cell permeability through PTP Shp-1 activity. (A) Quiescent hMVECs were pretreated with TIMP-2 (100nM) or Ala + TIMP-2 for 20 minutes and followed by VEGF-A (100 ng/mL) stimulation for 30 minutes. The results (mean ± SD) are expressed as the fold increase of FITC-dextran permeability in untreated control cells. **P < .01, compared with VEGF-A treatment alone. (B) Cells were pretreated with orthovanadate (2μM) for 20 minutes and followed by TIMP-2 or Ala + TIMP-2 before VEGF-A stimulation for 30 minutes. **P < .01, compared with VEGF-A treatment alone. (C) Cells transfected with control, Shp-1, or Shp-2 siRNA were treated as in panel A. **P < .01, compared with VEGF-A treatment alone. Cell lysates were Western blotted with anti-Shp-1, anti-Shp-2, or antiactin antibodies. Results are representative of 3 independent experiments.
Several PTPs have been previously implicated in regulating vascular permeability.1,4 Although Shp-1 has previously been implicated in the antiangiogenic effects of TIMP-2, unlike Shp-2 it has not previously been associated with regulation of vascular permeability.8,24,30 To determine whether Shp-1 also plays a role in TIMP-2 modulation of endothelial barrier function, we performed vascular permeability experiments using hMVECs transfected with siRNA of Shp-1 and Shp-2 as well as dn Shp-1 mutant. Shp-1 siRNA- and dn Shp-1 mutant-transduced cells, but not Shp-2 siRNA, completely reversed the suppressive effect of Ala + TIMP-2 on VEGF-A–induced increase in vascular permeability (Figure 1C; supplemental Figure 1A). Collectively, the findings presented in Figure 1 suggest that TIMP-2 suppresses the VEGF-A–induced increase in vascular permeability via an MMP-independent mechanism and that Shp-1 activity is specifically required for this effect.
TIMP-2 stimulates the production of cAMP in human microvascular endothelial cells, independent of MMP inhibitory activity
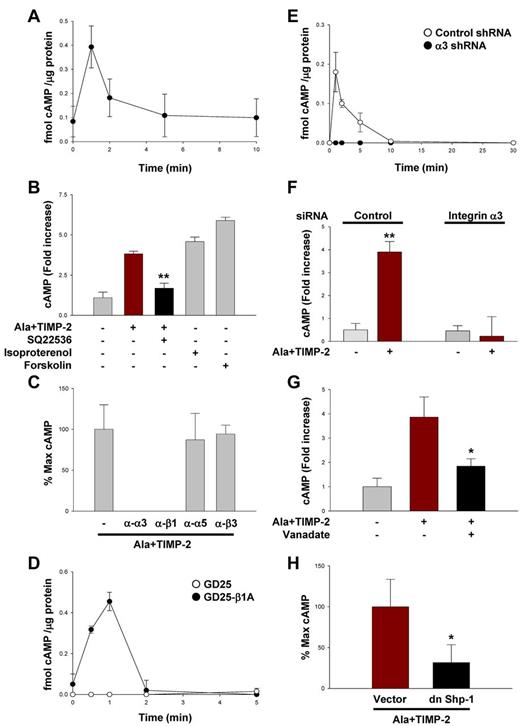
It is well known that elevated cyotosolic cAMP levels are protective of endothelial barrier function (reduced vascular permeability).4,16,17,38,40 The kinetics of cytosolic cAMP levels after treatment of quiescent hMVECs with TIMP-2 alone demonstrated a 4-fold increase over basal levels as early as 1 minute (Figure 2A), which rapidly returned to basal levels by 5 minutes. However, this TIMP-2–induced increase in cAMP did not alter the proliferative response of hMVECs under basal conditions similar to previous reports.26,31 Ala + TIMP-2 treatment also markedly stimulated the cAMP production to levels similar to those observed with TIMP-2–treated hMVECs (Figure 2A-B); however, pretreatment of hMVECs with the AC inhibitor SQ22536 (100μM) significantly abrogated the effect of Ala + TIMP-2 on cAMP production. In addition, hMVECs responded to cAMP-elevating reagents, such as isoproterenol (10μM) or forskolin (20μM), with similar elevation of cytosolic cAMP levels as observed after Ala + TIMP-2 treatment. Our observations demonstrate that TIMP-2 stimulates cytosolic cAMP levels in hMVECs through an MMP-independent mechanism that appears to involve activation of AC (blocked by SQ22536) and results in cAMP levels similar to those produced by pharmacologic agents known to be protective of endothelial barrier function.4,16,17,38,40 Because both TIMP-2 and Ala + TIMP-2 stimulated cAMP to similar levels, Ala + TIMP-2 was used in many subsequent experiments to focus on MMP-independent effects of TIMP-2.
TIMP-2 induces cAMP production in hMVECs, independent of MMP inhibitory activity. (A) Quiescent hMVECs were treated with TIMP-2 (100nM) for the indicated time points. The cAMP levels were measured as described in “cAMP determination.” Values represent the mean ± SD of 3 independent experiments. (B) Cells were pretreated with SQ22536 (100μM) for 20 minutes, followed by Ala + TIMP-2 for 1 minute, or treated with isoproterenol (10μM) and forskolin (20μM) for 1 minute. The results (mean ± SD) are expressed as the fold increase of cAMP levels in untreated cells. **P < .01, compared with Ala + TIMP-2 treatment alone. (C) Cells were pretreated with function-blocking anti-integrin antibodies (10 μg/mL) for 20 minutes, followed by Ala + TIMP-2 (100nM) for 1 minute. (D) Both GD25 and GD25-β1A MEFs were treated with Ala + TIMP-2 for the indicated time points. (E) A549 cells transfected with control or integrin α3 shRNA were treated with Ala + TIMP-2 as in panel D. The results from 3 independent experiments (mean ± SD) are presented as the percentage of maximally induced cAMP levels by Ala + TIMP-2 in the absence of integrin blocking antibodies or femtomoles of cAMP per microgram of protein in the samples to allow comparison between experiments. (F) Quiescent hMVECs transfected with control or integrin α3 siRNA were treated with Ala + TIMP-2 as in panel E. The results (mean ± SD) are expressed as the fold increase of cAMP levels in untreated cells. **P < .01, compared with Ala + TIMP-2 treatment alone. (G) Cells were pretreated with orthovanadate (2μM) for 20 minutes, followed by Ala + TIMP-2 for 1 minute. *P < .05, compared with Ala + TIMP-2 treatment alone. (H) Vector control and dn Shp-1 mutant-transduced hMVECs were treated with Ala + TIMP-2 for 1 minute. The results (mean ± SD) are presented as the percentage of maximal TIMP-2–induced cAMP levels in control vector cells (observed 1 minute after treatment). *P < .05, compared with Ala + TIMP-2 treatment alone in vector control cells.
TIMP-2 induces cAMP production in hMVECs, independent of MMP inhibitory activity. (A) Quiescent hMVECs were treated with TIMP-2 (100nM) for the indicated time points. The cAMP levels were measured as described in “cAMP determination.” Values represent the mean ± SD of 3 independent experiments. (B) Cells were pretreated with SQ22536 (100μM) for 20 minutes, followed by Ala + TIMP-2 for 1 minute, or treated with isoproterenol (10μM) and forskolin (20μM) for 1 minute. The results (mean ± SD) are expressed as the fold increase of cAMP levels in untreated cells. **P < .01, compared with Ala + TIMP-2 treatment alone. (C) Cells were pretreated with function-blocking anti-integrin antibodies (10 μg/mL) for 20 minutes, followed by Ala + TIMP-2 (100nM) for 1 minute. (D) Both GD25 and GD25-β1A MEFs were treated with Ala + TIMP-2 for the indicated time points. (E) A549 cells transfected with control or integrin α3 shRNA were treated with Ala + TIMP-2 as in panel D. The results from 3 independent experiments (mean ± SD) are presented as the percentage of maximally induced cAMP levels by Ala + TIMP-2 in the absence of integrin blocking antibodies or femtomoles of cAMP per microgram of protein in the samples to allow comparison between experiments. (F) Quiescent hMVECs transfected with control or integrin α3 siRNA were treated with Ala + TIMP-2 as in panel E. The results (mean ± SD) are expressed as the fold increase of cAMP levels in untreated cells. **P < .01, compared with Ala + TIMP-2 treatment alone. (G) Cells were pretreated with orthovanadate (2μM) for 20 minutes, followed by Ala + TIMP-2 for 1 minute. *P < .05, compared with Ala + TIMP-2 treatment alone. (H) Vector control and dn Shp-1 mutant-transduced hMVECs were treated with Ala + TIMP-2 for 1 minute. The results (mean ± SD) are presented as the percentage of maximal TIMP-2–induced cAMP levels in control vector cells (observed 1 minute after treatment). *P < .05, compared with Ala + TIMP-2 treatment alone in vector control cells.
Integrin α3β1 and Shp-1 activity is required for TIMP-2 induction of cAMP production
TIMP-2 inhibition of VEGF-A– or FGF-2–induced endothelial cell proliferation is mediated by binding to integrin α3β1, induction and translocation of Shp-1, which dephosphorylates RTKs.24,31 Pretreatment of quiescent hMVECs with anti-α3 or anti-β1 blocking antibodies, but not anti-α5 or anti-β3 blocking antibodies, completely abrogated the Ala + TIMP-2–mediated increase in cytosolic cAMP production (Figure 2C). Alternatively, we studied the effects of TIMP-2 on cAMP production in integrin β1-deficient murine embryonic fibroblasts (MEFs), and integrin α3 shRNA-transfected lung cancer cells. Integrin β1-deficient MEFs (GD25) were resistant to Ala + TIMP-2 (100nM) induction of cAMP levels (Figure 2D). In contrast, GD25-β1A cells (with reconstituted β1 subunit expression) responded to Ala + TIMP-2 (100nM) treatment with an ∼ 6-fold increase in cAMP levels and with similar kinetics as previously observed in hMVECs (Figure 2A). These results suggest that integrin β1 is required for TIMP-2 induction of cytosolic cAMP levels.
Inactivation of integrin β1 in the homozygous deficient mouse also results in suppression of cell surface expression of the integrin α3, α5, and α6 subunits.41 We next examined the effect of TIMP-2 on cAMP production in integrin α3-silenced A549 cells and hMVECs. Selective silencing of integrin α3 subunit completely abrogated the ability of TIMP-2 to induce cAMP levels in both cells (Figure 2E-F). Collectively, these findings from studies using integrin blocking antibodies, β1-deficient MEFs, and integrin α3 siRNA-transfected cells clearly demonstrate that integrin α3β1 is specifically required for TIMP-2–mediated increase in cytosolic cAMP production.
Pretreatment of hMVECs with orthovanadate (2μM), before Ala + TIMP-2 treatment, resulted in a marked reduction (∼ 65% decrease after correction for control levels) in TIMP-2–induced cAMP levels at 1 minute after treatment (Figure 2G). The specific requirement for Shp-1 activity in TIMP-2–mediated cAMP production was examined in dn Shp-1 mutant-transduced hMVECs. Ala + TIMP-2 treatment of vector control hMVECs resulted in the maximal induction of cAMP levels at 1 minute to similar levels observed in previous experiments (Figure 2H). However, cytosolic cAMP levels in dn Shp-1 mutant-transduced hMVECs were not increased after Ala + TIMP-2 treatment. These findings suggest that, like the reduction of VEGF-A–induced vascular permeability observed previously, Shp-1 activity is necessary for TIMP-2 increase in cytosolic cAMP levels and implies a functional relationship between TIMP-2 induction of Shp-1 and subsequent elevation of cAMP levels.
TIMP-2 promotes association of VE-cadherin with the cytoskeleton and reduced VEGF-A–induced VE-cadherin phosphorylation
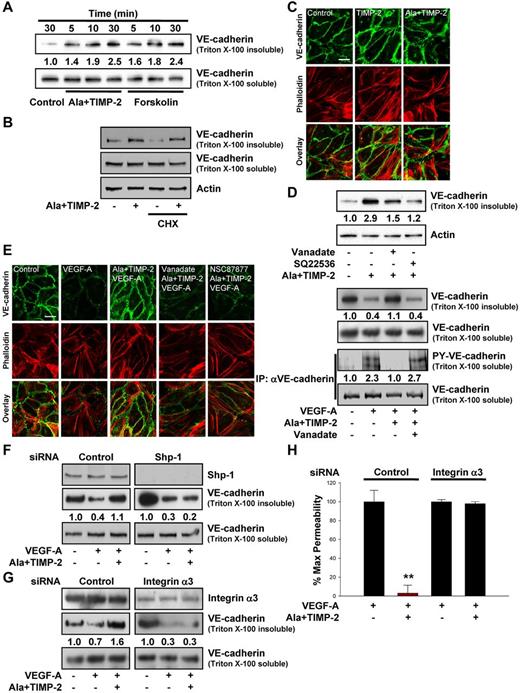
Elevation of cytosolic cAMP reportedly enhances the stability of adherens junctions, stabilization of VE-cadherin at cell-cell junctions, and maintains the barrier function of endothelial monolayers.4,17,38,40 Stimulation of endothelial cells with VEGF-A rapidly induces the dissociation of VE-cadherin from β-catenin and plakoglobin and disrupts association with the actin cytoskeleton. This alteration in VE-cadherin association with the cytoskeleton can be detected as a decrease of VE-cadherin detectable in the Triton X-100 insoluble cell extracts.35,36 Examination of the TIMP-2–mediated changes in the distribution of VE-cadherin in the Triton-insoluble compartment after treatment with Ala + TIMP-2 revealed redistribution of VE-cadherin into the Triton-insoluble fraction (actin cytoskeleton-associated) that was readily detectable as early as 5 minutes and was further increased over the 30-minute duration of this experiment (Figure 3A). Forskolin similarly increased detection of VE-cadherin in the detergent insoluble fraction with levels and kinetics similar to those of Ala + TIMP-2. Neither TIMP-2 nor forskolin had a significant effect on the levels of VE-cadherin in the Triton-soluble compartments. Moreover, pretreatment with the protein synthesis inhibitor cycloheximide (10μM) did not alter the TIMP-2–mediated VE-cadherin distribution in Triton-insoluble fraction, demonstrating that TIMP-2 induces VE-cadherin redistribution in Triton-insoluble compartment (Figure 3B). Immunofluorescence confocal microscopy revealed that TIMP-2 and Ala + TIMP-2 induce a significant redistribution of VE-cadherin (Figure 3C) and β-catenin (supplemental Figure 1B) to cell-cell contacts and association with the cortical actin cytoskeleton (colocalization signal in merged VE-cadherin/phalloidin staining), in agreement with our observations from Western blotting of VE-cadherin of Triton X-100–insoluble fractions (Figure 3A).
TIMP-2 regulation of VE-cadherin distribution and endothelial cell permeability is mediated by an integrin α3β1-Shp-1-cAMP–dependent pathway. (A) Quiescent hMVECs were treated with Ala + TIMP-2 (100nM) or forskolin (10μM) for the indicated time points. Translocation of VE-cadherin was assessed by Triton X-100 solubility. The Triton X-100 insoluble or soluble fraction was resolved by SDS-PAGE and Western blotted with anti–VE-cadherin antibodies. The integrated band intensities for the VE-cadherin (Triton-insoluble fraction) were normalized to untreated control cells at 30 minutes. Results are representative of 3 independent experiments. (B) Cells were treated with cycloheximide (CHX, 10μM) for 1 hour, followed by Ala + TIMP-2 for 30 minutes. Cell lysates were Western blotted with anti–VE-cadherin and antiactin antibodies. (C) Cells were treated with TIMP-2 or Ala + TIMP-2 for 30 minutes, and colocalization of VE-cadherin and actin was determined as described in “Immunofluorescence microscopy.” Scale bar represents 10 μm. (D) Cells were pretreated with orthovanadate (2μM) or SQ22536 (100μM) for 20 minutes and followed by Ala + TIMP-2 for 30 minutes. Results are representative of 3 independent experiments. (E) Cells were pretreated with orthovanadate or NSC87877 (2μM) for 20 minutes and followed by Ala + TIMP-2 (100nM) before VEGF-A (100 ng/mL) stimulation for 30 minutes, and assessed by confocal microscopy (left panel) and Western blot analysis (right panel). The Triton-insoluble fraction was Western blotted with anti–VE-cadherin antibodies. Anti-VE-cadherin immunoprecipitates (IP) were Western blotted with anti-phosphotyrosine or anti–VE-cadherin antibodies. Results are representative of 3 independent experiments. (F-G) Cells transfected with control, Shp-1, or integrin α3 siRNA were pretreated with Ala + TIMP-2 and followed by VEGF-A for 30 minutes. The Triton-insoluble or soluble fraction was Western blotted with anti-Shp-1, anti-integrin α3, or anti–VE-cadherin antibodies. Integrated density values were normalized to untreated controls. Results are representative of 3 independent experiments. (H) In vitro endothelial cell permeability was performed as described in “Permeability assay.” The results from 3 independent experiments (mean ± SD) are presented as the percentage of maximally induced permeability by VEGF-A in the absence of Ala + TIMP-2. **P < .01, compared with VEGF-A treatment alone.
TIMP-2 regulation of VE-cadherin distribution and endothelial cell permeability is mediated by an integrin α3β1-Shp-1-cAMP–dependent pathway. (A) Quiescent hMVECs were treated with Ala + TIMP-2 (100nM) or forskolin (10μM) for the indicated time points. Translocation of VE-cadherin was assessed by Triton X-100 solubility. The Triton X-100 insoluble or soluble fraction was resolved by SDS-PAGE and Western blotted with anti–VE-cadherin antibodies. The integrated band intensities for the VE-cadherin (Triton-insoluble fraction) were normalized to untreated control cells at 30 minutes. Results are representative of 3 independent experiments. (B) Cells were treated with cycloheximide (CHX, 10μM) for 1 hour, followed by Ala + TIMP-2 for 30 minutes. Cell lysates were Western blotted with anti–VE-cadherin and antiactin antibodies. (C) Cells were treated with TIMP-2 or Ala + TIMP-2 for 30 minutes, and colocalization of VE-cadherin and actin was determined as described in “Immunofluorescence microscopy.” Scale bar represents 10 μm. (D) Cells were pretreated with orthovanadate (2μM) or SQ22536 (100μM) for 20 minutes and followed by Ala + TIMP-2 for 30 minutes. Results are representative of 3 independent experiments. (E) Cells were pretreated with orthovanadate or NSC87877 (2μM) for 20 minutes and followed by Ala + TIMP-2 (100nM) before VEGF-A (100 ng/mL) stimulation for 30 minutes, and assessed by confocal microscopy (left panel) and Western blot analysis (right panel). The Triton-insoluble fraction was Western blotted with anti–VE-cadherin antibodies. Anti-VE-cadherin immunoprecipitates (IP) were Western blotted with anti-phosphotyrosine or anti–VE-cadherin antibodies. Results are representative of 3 independent experiments. (F-G) Cells transfected with control, Shp-1, or integrin α3 siRNA were pretreated with Ala + TIMP-2 and followed by VEGF-A for 30 minutes. The Triton-insoluble or soluble fraction was Western blotted with anti-Shp-1, anti-integrin α3, or anti–VE-cadherin antibodies. Integrated density values were normalized to untreated controls. Results are representative of 3 independent experiments. (H) In vitro endothelial cell permeability was performed as described in “Permeability assay.” The results from 3 independent experiments (mean ± SD) are presented as the percentage of maximally induced permeability by VEGF-A in the absence of Ala + TIMP-2. **P < .01, compared with VEGF-A treatment alone.
Pretreatment of quiescent hMVECs with orthovanadate or SQ22536 before Ala + TIMP-2 markedly reduced the redistribution of VE-cadherin into Triton-insoluble compartments by > 50%-75%, respectively (Figure 3D). These findings correlate well with the effects of orthovanadate on TIMP-2 reduction of VEGF-A–mediated increase in vascular permeability (Figure 1B) and blockade of TIMP-2 increase in cytosolic cAMP levels (Figure 2G) observed previously.
Previous reports show that vascular permeability can be modulated by the phosphorylation of VE-cadherin as well as the changes of VE-cadherin cytoskeletal association.4,10 VEGF-A induced dramatic loss of VE-cadherin and β-catenin in Triton-insoluble compartment (supplemental Figure 1D) and reduced the association of VE-cadherin and β-catenin with the cytoskeleton as shown by a decrease at cell-cell contacts (Figure 3E left panel; supplemental Figure 1C) and in the Triton-insoluble fraction (Figure 3E right panel, upper 2 blots), compared with untreated controls. Consistent with this observation, VEGF-A stimulated the tyrosine phosphorylation of VE-cadherin and reduced the association of α-catenin and γ-catenin with VE-cadherin in Triton-soluble compartment (Figure 3E right panel, lower 2 blots; supplemental Figure 1E). Treatment with Ala + TIMP-2 prevented the VEGF-A–induced loss of VE-cadherin and β-catenin from the cell-cell contacts (Figure 3E; supplemental Figure 1C) while concomitantly abrogating VEGF-A–induced increases in the level of tyrosine phosphorylated VE-cadherin and dissociation of α-catenin and γ-catenin from VE-cadherin in the soluble compartment (Figure 3E right panel, bottom 2 blots; supplemental Figure 1E). Both the TIMP-2–mediated increase in the association of VE-cadherin and β-catenin with cytoskeleton (increase in Triton-insoluble fraction) as well as the decrease in tyrosine phosphorylated VE-cadherin in the Triton-soluble compartment were reversed by orthovanadate or NSC87877, implicating a role for Shp-1 activity. In contrast, the levels of β-catenin, γ-catenin, and p120-catenin show little or no change after VEGF-A treatment in the absence or presence of TIMP-2 (supplemental Figure 1F). The Triton-insoluble compartment did not show detectable phosphorylation of VE-cadherin (data not shown), consistent with previous reports in which VE-cadherin phosphorylation is associated with impaired barrier function.4,42 Furthermore, TIMP-2 induced the association of VE-cadherin with VEGFR-2, but not integrin α3β1 (data not shown), which is dependent on Shp-1 activity (supplemental Figure 2).
Selective silencing of Shp-1 gene expression in hMVECs using siRNA-targeting demonstrated that Shp-1 siRNA successfully reduced expression of Shp-1 (> 90% decrease) compared with the controls (Figure 3F). Ala + TIMP-2 treatment prevented the VEGF-A–induced redistribution of VE-cadherin out of the Triton-insoluble compartment of control siRNA-transfected hMVECs (Figure 3F). In contrast, Ala + TIMP-2 treatment failed to prevent the VEGF-A–induced redistribution of VE-cadherin out of the Triton-insoluble compartment in Shp-1 siRNA-transfected cells. These results suggest that, like the TIMP-2 preservation of endothelial barrier function after VEGF-A stimulation (Figure 1B-C), VE-cadherin cytoskeletal association can be regulated in an Shp-1–dependent fashion.
TIMP-2 regulation of VE-cadherin distribution and endothelial permeability is mediated by integrin α3β1
To confirm whether integrin α3β1 is required for TIMP-2 regulation of endothelial permeability, we examined the effects of TIMP-2 on VE-cadherin distribution and permeability in hMVECs transfected with integrin α3 siRNA. As shown in Figure 3G, introduction of the α3 siRNA significantly reduced α3 expression, as observed in whole cell lysates. Suppression of α3 integrin subunit expression also abrogated the Ala + TIMP-2–mediated increase of VE-cadherin in the Triton-insoluble compartment after VEGF-A stimulation (Figure 3G), and integrin α3 siRNA-transfected cells were completely unresponsive to Ala + TIMP-2 inhibition of vascular permeability (Figure 3H). This is similar to the effects of anti-α3 blocking antibodies and α3 siRNA on TIMP-2–stimulated increase in cytosolic cAMP levels observed previously (Figure 2C,E-F), again suggesting a functional relationship between an increase in cAMP and enhanced endothelial barrier function. Taken together, the data in Figure 3 provide a functional linkage between Shp-1–dependent, TIMP-2–mediated decrease in vascular permeability, induction of cytosolic cAMP levels, reduced VE-cadherin phosphorylation, and enhanced VE-cadherin interaction with the cytoskeleton.
PKA inhibitor abrogates TIMP-2 increase of VE-cadherin cytoskeletal association
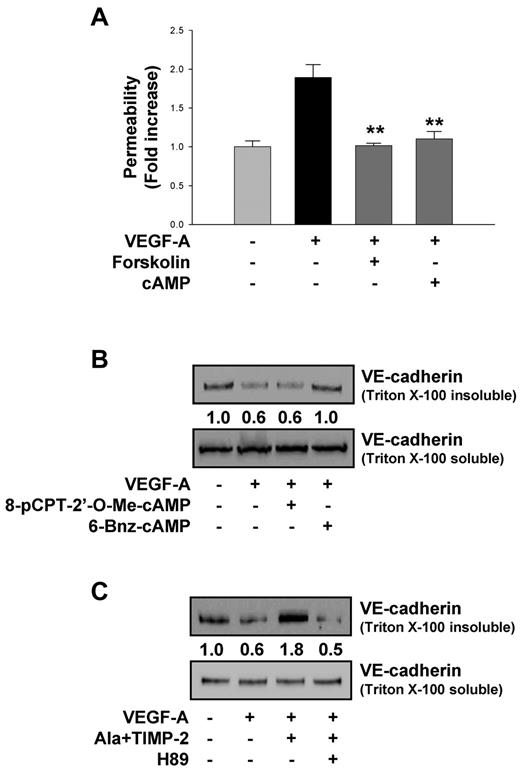
It has long been known that cAMP-elevating agents can regulate vascular permeability.4,40 As shown in Figure 4A, forskolin (10μM) or the nonhydrolyzable cAMP analog dibutyryl cAMP (1mM) mimicked the suppressive effects of TIMP-2 on VEGF-A–induced vascular permeability (Figure 1). These results suggest that TIMP-2 may regulate VEGF-A–induced vascular permeability via a cAMP-dependent mechanism. It is also well known that cytosolic cAMP activates 2 downstream effectors Epac and PKA, both of which are known to enhance endothelial barrier function, and it may be that the specific downstream effector is dependent on the endothelial cell origin.16-18,38,43,44 To examine which of these downstream effector pathways mediates TIMP-2 suppression of VEGF-A–induced increase in vascular permeability, we used both a PKA-specific activator, 6-Bnz-cAMP, and an Epac-specific activator, 8-pCPT-2′-O-Me-cAMP. Treatment of hMVECs with 6-Bnz-cAMP before VEGF-A stimulation increased VE-cadherin distribution in Triton-insoluble compartments; however, 8-pCPT-2′-O-Me-cAMP did not alter the distribution of VE-cadherin (Figure 4B), suggesting that in hMVECs barrier function (decreased permeability) is preserved after VEGF-A stimulation via a PKA-dependent mechanism. Consistent with this observation, we demonstrate that the PKA inhibitor H89 completely abrogates the ability of TIMP-2 to increase VE-cadherin distribution in Triton-insoluble compartments, demonstrating that PKA activity mediates the TIMP-2 induction of VE-cadherin association with the cytoskeleton (Figure 4C).
TIMP-2 modulates VEGF-A–induced VE-cadherin distribution through PKA activity. (A) Quiescent hMVECs were pretreated with forskolin (10μM) or nonhydrolyzable cAMP analog, dibutyryl cAMP (1mM) for 20 minutes and followed by VEGF-A (100 ng/mL) stimulation for 30 minutes. Values represent the mean ± SD of 3 independent experiments. **P < .01, compared with VEGF-A treatment alone. (B) Cells were pretreated with 6-Bnz-cAMP (100μM) or 8-pCPT-2′-O-Me-cAMP, and stimulated with VEGF-A for 30 minutes. The Triton-insoluble or soluble fraction was Western blotted with anti–VE-cadherin antibodies. (C) Cells were pretreated with H89 (20μM) for 20 minutes and followed by Ala + TIMP-2 (100nM) before VEGF-A stimulation for 30 minutes. Integrated density values were obtained and normalized to untreated cells. Results are representative of 3 independent experiments.
TIMP-2 modulates VEGF-A–induced VE-cadherin distribution through PKA activity. (A) Quiescent hMVECs were pretreated with forskolin (10μM) or nonhydrolyzable cAMP analog, dibutyryl cAMP (1mM) for 20 minutes and followed by VEGF-A (100 ng/mL) stimulation for 30 minutes. Values represent the mean ± SD of 3 independent experiments. **P < .01, compared with VEGF-A treatment alone. (B) Cells were pretreated with 6-Bnz-cAMP (100μM) or 8-pCPT-2′-O-Me-cAMP, and stimulated with VEGF-A for 30 minutes. The Triton-insoluble or soluble fraction was Western blotted with anti–VE-cadherin antibodies. (C) Cells were pretreated with H89 (20μM) for 20 minutes and followed by Ala + TIMP-2 (100nM) before VEGF-A stimulation for 30 minutes. Integrated density values were obtained and normalized to untreated cells. Results are representative of 3 independent experiments.
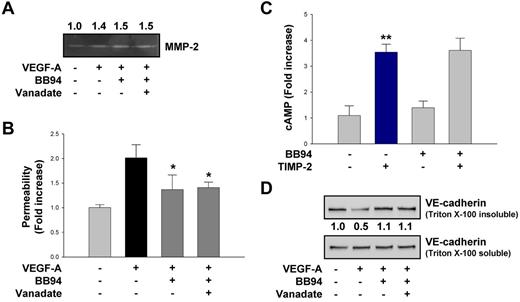
Effect of MMP inhibitors on VEGF-A–induced increase in vascular permeability is distinct from TIMP-2
MMP-mediated proteolytic shedding of VE-cadherin has been shown by use of synthetic MMP inhibitors.4,12,45 Treatment of VEGF-A–stimulated hMVECs with either the synthetic broad-spectrum MMP inhibitor BB94 (100nM) or orthovanadate (2μM) did not alter the 50% increase in MMP-2 expression observed after VEGF-A stimulation (Figure 5A). However, BB94 treatment resulted in an ∼ 50% reduction in the VEGF-A–induced increase in permeability in vitro (Figure 5B), extending previous observations that MMP-2 can cleave VE-cadherin and alter endothelial permeability.45 Pretreatment of hMVECs with BB94 (100nM) did not alter cAMP levels compared with untreated cells or alter the cAMP response observed in TIMP-2–treated cells (Figure 5C) but did prevent the VEGF-A–induced decrease in VE-cadherin detectable in the Triton-insoluble compartment (Figure 5D). These findings are consistent with a previous report that MMP-2 can cleave VE-cadherin, resulting in an increase of endothelial permeability, but was blocked by synthetic MMP inhibitors.45 However, orthovanadate pretreatment failed to alter BB-94–induced decrease in endothelial permeability or increase in VE-cadherin cytoskeletal response to VEGF-A stimulation (Figure 5B,D), thus distinguishing this response from that observed after TIMP-2 treatment. These findings suggest that MMP-inhibitory activity regulates VEGF-A–induced permeability via a mechanism that is not a potent as TIMP-2, does not involve cAMP or Shp-1, and probably involves direct inhibition of VE-cadherin cleavage.
An MMP inhibitor regulates VEGF-A–induced VE-cadherin distribution and endothelial cell permeability but does not require PTP activity and cAMP production. (A) Quiescent hMVECs were pretreated with orthovanadate (2μM) for 20 minutes and followed by BB94 (100nM) before VEGF-A (100 ng/mL) stimulation for 30 minutes. Zymogram analysis results are representative of 3 independent experiments. (B) Cells were treated as in panel A, and vascular permeability was measured as described. Values represent the mean ± SD of 3 independent experiments. *P < .05, compared with VEGF-A treatment alone. (C) Cells were pretreated with BB94 for 20 minutes, followed by TIMP-2 (100nM) treatment for 1 minute. The results (mean ± SD) are expressed as the fold increase of cAMP levels in untreated cells. **P < .01, compared with untreated control. (D) Cells were treated as in panel A, and the Triton-insoluble or soluble fraction was Western blotted with anti–VE-cadherin antibodies. Integrated density values were obtained and normalized to untreated controls. Results are representative of 3 independent experiments.
An MMP inhibitor regulates VEGF-A–induced VE-cadherin distribution and endothelial cell permeability but does not require PTP activity and cAMP production. (A) Quiescent hMVECs were pretreated with orthovanadate (2μM) for 20 minutes and followed by BB94 (100nM) before VEGF-A (100 ng/mL) stimulation for 30 minutes. Zymogram analysis results are representative of 3 independent experiments. (B) Cells were treated as in panel A, and vascular permeability was measured as described. Values represent the mean ± SD of 3 independent experiments. *P < .05, compared with VEGF-A treatment alone. (C) Cells were pretreated with BB94 for 20 minutes, followed by TIMP-2 (100nM) treatment for 1 minute. The results (mean ± SD) are expressed as the fold increase of cAMP levels in untreated cells. **P < .01, compared with untreated control. (D) Cells were treated as in panel A, and the Triton-insoluble or soluble fraction was Western blotted with anti–VE-cadherin antibodies. Integrated density values were obtained and normalized to untreated controls. Results are representative of 3 independent experiments.
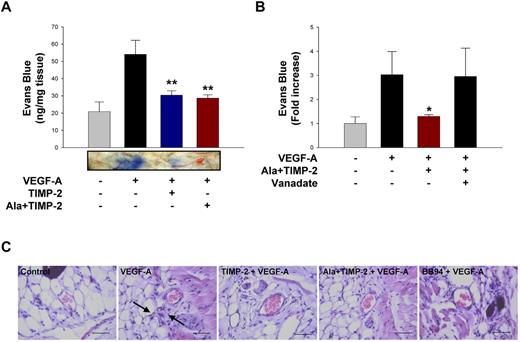
TIMP-2 and Ala + TIMP-2 prevent VEGF-A–induced increase in vascular permeability in vivo
The effect of TIMP-2 and Ala + TIMP-2 on VEGF-A–induced vascular permeability was measured in vivo using the Miles assay. Both TIMP-2 and Ala + TIMP-2 inhibited VEGF-A–mediated increase in vascular permeability with similar potency (∼ 70% inhibition; Figure 6A). In addition, the in vivo effect of Ala + TIMP-2 is orthovanadate sensitive (Figure 6B). The finding that the in vivo increase in vascular permeability after local intradermal injection of VEGF-A is significantly reduced (P < .01) by coadministration of Ala + TIMP-2 suggests that this effect, like the in vitro effect, is independent of MMP-inhibitory activity but dependent on PTP activity. Microscopic examination of hematoxylin and eosin-stained sections showed that VEGF-A treatment induced the extravasation of blood cells into peripheral tissues; however, TIMP-2 and Ala + TIMP-2 as well as BB94 efficiently prevented the VEGF-A–induced responses (Figure 6C).
TIMP-2 and Ala + TIMP-2 inhibit VEGF-A–induced vascular permeability in vivo through PTP activity. (A) Quantification of in vivo permeability was performed using the modified Miles assay. Mice were injected intradermally with 50 μL of VEGF-A (1 μg/mL) with TIMP-2 (500nM) or Ala + TIMP-2. The results (mean ± SE) from 3 independent experiments are presented as nanograms of weight of extravasated Evans blue dye per milligram of weight of dermal tissue sample. **P < .01, compared with VEGF-A treatment alone. Representative Miles assay results are shown below the graph. (B) Mice were injected with VEGF-A with Ala + TIMP-2 or orthovanadate (5μM). The results (mean ± SE) from 4 independent experiments are presented as the fold increase of in vivo permeability in untreated controls. The amount of extravasated Evans blue dye in untreated controls was 18 ± 5.6 ng/mg tissue. *P < .05, compared with VEGF-A treatment alone. (C) Mice (5 mice in each group) were treated as in panel A, and tissue sections were analyzed by hematoxylin and eosin staining (original magnification ×400). Scale bars represent 50 μm. The black arrows indicate the extravasation of blood cells.
TIMP-2 and Ala + TIMP-2 inhibit VEGF-A–induced vascular permeability in vivo through PTP activity. (A) Quantification of in vivo permeability was performed using the modified Miles assay. Mice were injected intradermally with 50 μL of VEGF-A (1 μg/mL) with TIMP-2 (500nM) or Ala + TIMP-2. The results (mean ± SE) from 3 independent experiments are presented as nanograms of weight of extravasated Evans blue dye per milligram of weight of dermal tissue sample. **P < .01, compared with VEGF-A treatment alone. Representative Miles assay results are shown below the graph. (B) Mice were injected with VEGF-A with Ala + TIMP-2 or orthovanadate (5μM). The results (mean ± SE) from 4 independent experiments are presented as the fold increase of in vivo permeability in untreated controls. The amount of extravasated Evans blue dye in untreated controls was 18 ± 5.6 ng/mg tissue. *P < .05, compared with VEGF-A treatment alone. (C) Mice (5 mice in each group) were treated as in panel A, and tissue sections were analyzed by hematoxylin and eosin staining (original magnification ×400). Scale bars represent 50 μm. The black arrows indicate the extravasation of blood cells.
Discussion
MMPs are capable of degrading all components of the extracellular matrix as well as an array of cell surface molecules, cell adhesion receptors, and pericellular proteins that have profound influences on cell behavior, tissue development, and disease progression. TIMPs are physiologic inhibitors of MMP activity, and it is usually assumed that TIMP influences on cell function, tissue assembly, and disease states occur principally through inhibition of MMP activity. However, a growing body of evidence from a number of independent laboratories suggests that TIMPs may also regulate cell behavior independent of MMP inhibitory activity via specific surface receptor-initiated cell signaling pathways.23-25,27,46
TIMP-2 is the only member of the TIMP family that has been shown to selectively inhibit endothelial cell growth and angiogenesis in an MMP-independent fashion.24,25,29-31 We have shown previously that TIMP-2 reduced phosphorylation of several RTKs after stimulation with their cognate ligands through an increase of Shp-1 with the receptors.24,30,31,46 This results in a selective alteration in the RTK phosphorylation pattern with subsequent consequences in downstream signaling pathways involving a variety of cellular responses.46 In the present study, we demonstrate that TIMP-2 modulates the earliest event of the angiogenic response to VEGF-A both in vitro and in vivo, specifically, increased vascular permeability. TIMP-2 effectively blocks the increase in vascular permeability observed after VEGF-A treatment. The in vitro mechanism of this effect requires integrin α3β1 for induction of Shp-1 activity as well as an increase in cytosolic cAMP and downstream PKA activity. These findings are evidenced by genetic silencing of integrin α3 or Shp-1, expression of dn Shp-1, and by pretreatment with PTP, AC, or PKA inhibitors, all of which disrupted the collective effects of TIMP-2/Ala + TIMP-2 on preservation of hMVEC barrier function and VE-cadherin cytoskeletal association, which are disrupted after VEGF-A stimulation. We demonstrated, for the first time, that both TIMP-2 and Ala + TIMP-2, a mutant form of TIMP-2 without MMP inhibitory activity, induced cAMP production in hMVEC in a manner that requires Shp-1. TIMP-2 markedly increased VE-cadherin association with the cytoskeleton and distribution at cell-cell contacts, which was abrogated by pretreatment with Shp-1 inhibitor NSC87877 (or orthovanadate), AC inhibitor SQ22536, and PKA inhibitor H89. Furthermore, the Miles assay and tissue histology study demonstrated that VEGF-A–induced increase in vascular permeability was decreased by TIMP-2 or Ala + TIMP-2. Consistent with our in vitro experiments, these in vivo effects were also reversed by orthovanadate and also corroborate previous findings that the effects of TIMP-2 on endothelial cell proliferation and angiogenesis in vivo require PTP activity.24,30
We have previously reported that TIMP-2 treatment of hMVECs results in activation of c-terminal src kinase activity and a decrease in src kinase.34 Although it is known that src family kinases down-regulate endothelial barrier function, it does not appear that TIMP-2 preservation of barrier function is mediated by inhibition of src kinase but is more likely because of up-regulation of Shp-1 phosphatase, as the Shp-1 inhibitor, suppression of Shp-1, and expression of dn Shp-1 disrupt TIMP-2 barrier preservation. TIMP-2 shares the ability to activate this Shp-1 pathway with another angiostatic agent that binds to an integrin receptor, endorepellin.47 Endorepellin has been shown to interact with integrin α2β1, induce cAMP, as well as activate PKA and Shp-1.47,48 As previously observed with TIMP-2, this leads to inhibition of endothelial cell growth, migration, and Shp-1–dependent dephosphorylation of RTKs. Based on current findings, one might predict that endorepellin would also function to preserve endothelial barrier function, but this has not yet been demonstrated. The association of Shp-1 activity and PKA in suppressing endothelial growth and migration by both TIMP-2 and endorepellin has led to the suggestion that PKA may regulate Shp-1 by phosphorylating serine residue in the c-terminal domain, creating a potential autoregulatory loop.47 However, the function and physiologic significance of this potential mechanism remain to be demonstrated. Integrin ligand binding and activation of PTP activity, including Shp-1, appears to be an emerging pathway for regulation of RTK-mediated cellular responses.49,50
MMPs reportedly cleave VE-cadherin ectodomain and disrupt endothelial cell contacts, effects that can be blocked by broad-spectrum MMP inhibitors.12,45 The current study extends these findings by demonstrating that a broad-spectrum MMP inhibitor, BB94, can indeed modestly block VEGF-A–induced increase in vascular permeability and VE-cadherin cytoskeletal association in vitro. However, this effect was distinct from the observed effects of TIMP-2 and/or Ala + TIMP-2 on endothelial barrier function in that the effects of TIMP-2 are clearly dependent on cell signaling and can be disrupted by inhibition of Shp-1, AC, or PKA activity. In contrast, the effect of BB94 is not dependent on increased cytosolic cAMP, is not inhibited by the PTP inhibitor orthovanadate, but is consistent with inhibition of MMP-mediated cleavage of VE-cadherin as previously reported.12,45
In the present report, we show that TIMP-2 inhibits VEGF-A–induced vascular permeability through a novel Shp-1-cAMP/PKA–dependent mechanism that results in increased association of VE-cadherin with the actin cytoskeleton and accumulation at cell-cell contacts. The results extend our previous observations on the antiangiogenic effects of TIMP-2 and describe what appears to be an emerging signaling paradigm involving integrin activation of Shp-1, cAMP, and PKA. Identification of TIMP-2 targets and further understanding the signaling mechanisms involved in mediating TIMP-2 antiangiogenic responses could provide novel therapeutic strategies for the treatment of the pathophysiologic consequences of VEGF-induced vascular permeability, including inflammatory and cardiovascular diseases, as well as cancer. Specifically, TIMP-2 could potentially improve drug delivery and/or radiosensitivity in tumors. These findings warrant the continued preclinical development of TIMP-2 as a novel antiangiogenic agent.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute, Center for Cancer Research Project (intramural research funds Z01SC 009179, W.G.S.-S.), and Basic Science Research Program through the National Research Foundation of Korea from the Ministry of Education, Science and Technology (KRF-2008-313-C00555 and 2010-0021913, D.-W.S.).
National Institutes of Health
Authorship
Contribution: S.H.K., Y.-R.C., and D.-W.S. designed and performed research and wrote the manuscript; H.-J.K., E.-K.A., H.-J. Ko, S.-J.L., and Y.C. performed research; J.S.O., B.J.H., and Y.K.K. analyzed data; and W.G.S.-S. and D.-W.S. supervised the entire project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William G. Stetler-Stevenson, Extracellular Matrix Pathology Section, Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Advanced Technology Center, Rm 115D, Bethesda, MD 20892-4605; e-mail: sstevenw@mail.nih.gov; and Dong-Wan Seo, Laboratory of Biochemistry, College of Pharmacy, Dankook University, Pharmacy Hall, Rm 319, Cheonan 330-714, Republic of Korea; e-mail: dwseomb@dankook.ac.kr.
References
Author notes
S.H.K. and Y.-R.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal