Abstract
Delays in immune recovery after allogeneic hematopoietic stem cell transplantation (allo-HSCT) are associated with increased risks of infection and relapse. IL-7 has a central role in T-cell development and survival and enhances immune recovery in murine models of allo-HSCT. We performed a phase 1 trial of r-hIL-7 (CYT107) in recipients of T-cell depleted allo-HSCTs. Twelve patients were treated with escalating doses of r-hIL-7 administered weekly for 3 weeks. The study drug was well tolerated with only one patient developing acute skin GVHD. At baseline, patients were profoundly lymphopenic. CYT107 induced a doubling in CD4+ and CD8+ T cells. The main effect of IL-7 was an expansion of effector memory T cells, the predominant subset identified in our patients. There was no significant effect on CD4+CD25+FoxP3+ T cells, NK, or B cells. Importantly, we not only saw quantitative increases in T cells after a short course of IL-7 but also demonstrated an increase in functional T cells, including viral-specific T cells that recognize CMV. Enhanced TCR diversity was also observed after treatment. Our results indicate that r-hIL-7 can enhance immune recovery after a T cell–depleted allo-HSCT without causing significant GVHD or other serious toxicity (www.clinicaltrials.gov; NCT00684008).
Introduction
Delays in immune recovery after allogeneic hematopoietic stem cell transplantation (allo-HSCT) are associated with increased risks of infection, relapse, and secondary malignancies.1-6 The risk of opportunistic infections is correlated with T-cell recovery, and in particular CD4+ T cells.1,7 Strategies to enhance T-cell reconstitution may therefore decrease posttransplantation morbidity and mortality. IL-7 has a central role in T-cell development and survival, and it enhances thymopoiesis and peripheral T-cell survival and expansion in murine models of allo-HSCT.8-19 Initial trials with recombinant human IL-7 (r-hIL-7) demonstrated an expansion of CD4+ and CD8+ T cells in patients with solid tumors or HIV infection.20-25 We conducted a phase 1 trial of r-hIL-7 (CYT107, Cytheris Inc) in recipients of T cell–depleted (TCD) allo-HSCTs and showed that r-hIL-7 was well tolerated and induced a rapid increase in peripheral CD4+ and CD8+ T cells.
Methods
Patient population
Patients more than 15 years of age were in remission without active or prior GVHD 60-210 days after TCD allo-HSCT from an 8 of 8 HLA-matched donor for treatment of nonlymphoid hematologic malignancy. The timing interval of study entry was selected to allow for adequate engraftment and the absence of significant transplant-related complications, as well as the inclusion of patients with poor immune recovery at 6 months after HSCT (CD4 < 100/mm3). Additional requirements included documented engraftment (sustained absolute neutrophil count > 1000/mm3, platelet count > 20 000/mm3), Karnofsky performance status > 60, normal left ventricular ejection fraction and pulmonary function, total bilirubin within 1.5 × the upper limit of normal, aspartate aminotransferase, and alanine aminotransferase within 2.5 × the upper limit of normal, prothrombin time/partial thromboplastin time within 1.5 × the upper limit of normal, creatinine clearance > 60 mL/min/1.73 m2. Exclusions were evidence or history of acute or chronic GVHD, relapsed disease, active uncontrolled infection, infections with HIV or hepatitis B or C, treatment with anticoagulants, systemic corticosteroids, cytotoxic or immunosuppressive therapy, growth hormone or gonadotropin agonists/antagonists, cytokine support other than G-CSF, uncontrolled hypertension, history of lymphoid malignancy or acute biphenotypic leukemia, peripheral lymphadenopathy, and history of autoimmune disease and severe uncontrolled asthma. The study was approved by the Institution Review Board and regulatory authorities. All patients gave informed consent in accordance with the Declaration of Helsinki. The study was registered at www.clinicaltrial.gov as NCT00684008.
Study design and treatment plan
The primary study objectives were safety, dose-limiting toxicity, and maximum tolerated dose determination. Toxicity was evaluated according to NCI Common Toxicity Criteria (Version 3.0). Secondary objectives included defining a range of biologically active doses based on T-cell recovery; pharmacokinetics; effects on engraftment, GVHD, Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder (EBV-PTLD), and relapse. Data were analyzed as of December 31, 2011.
Plasma anti–IL-7 antibody determination
Antibody detection used a 2-step ELISA and a bioassay for neutralization of IL-7 bioactivity, both developed for and used during r-hIL-7 preclinical development by Cytheris.25 Pretreatment and day 28 plasma samples were assayed. Assays were repeated at days 35 and 42 if equivocal at day 28. A titer > 1/200 was considered positive for the ELISA. A neutralizing antibody titer > 1/400 at either day 28 or 42 was considered positive and a dose-limiting toxicity.
Plasma r-hIL-7 level determination and pharmacokinetics
Plasma IL-7 levels were determined using a 2-site sandwich ELISA kit (Diaclone Research), modified to include CYT107 as the standard.25 The lower limit of quantification and the limit of detection was determined as 12.5 pg/mL and 3.125 pg/mL, respectively. Concentrations below the lower limit of quantification were treated as limit of detection (3.125 pg/mL) for the calculation of pharmacokinetic parameters (Kinetica Version 4.2 software). The linear relationship between r-hIL-7 concentrations and absorbance extended from 6.25 to 200 pg/mL. The noncompartmental extravascular template was used for evaluating the PK parameters. The area under the curve was computed using the log linear method, trapezoidal when Cn > Cn − 1. Half-lives were calculated between t = 2 hours (Tmax) and T = 24 hours.
Immune recovery
Circulating lymphocyte subsets were measured at baseline, 14, 21, 28, and 77 (± 7) days after the first injection, whereas T-cell proliferative responses (measured by 3H-TdR incorporation) to mitogens (PHA and OKT3), recall (candida, tetanus), viral (CMV, HSV, VZV, adenovirus), and allogeneic antigens were measured at baseline, 28 and 77 (± 7) days after the first injection, as previously published.1 Thawed PBMCs were stained using the following fluorochrome-labeled antibodies: allophycocyanin (APC)-CD45RA, PE-Cy7-CD3 (BD Biosciences); FITC-Ki67, PE-Bcl2, FITC-HLA-DR, PE-Cy5-CD8, APC-CD14, PE-Cy7-CD16, PC-C7-CD19, PE-CD25, APC-CD25, PE-CD27, peridin chlorophyll protein-Cy5.5-CD28, PE-CD31, APC-CD45RA, APC-CD56, FITC-CD95, and PE-CD103 (BD Biosciences PharMingen); AF405-CD3, AF405-CD8 (Caltag Laboratories), ECD-CD4, ECD-CD8, ECD-CD45RA (Beckman Coulter); Pacific Blue–CD3, APC-AF750-CD4, APC-AF750-CD8, APC-AF750-CD127, APC-AF750-CD62L, APC-FOXP3 (eBioscience); and FITC-CCR7 (R&D Systems). Cells were analyzed by flow cytometry using a CYAN flow cytometer with Summit software (DakoCytomation California). The percentage of positive cells was determined by gating on the population of cells that were viable (forward scatterlow and side scatterlow), CD3high and CD4high or CD8high.
Quantitation of CMV-specific T cells
CMV-specific T cells were quantified using an intracellular IFN-γ production assay and tetramer analysis.26,27 IFN-γ production by T cells stimulated with protein-spanning pools of CMV pp65–derived overlapping pentadecapeptides was performed by culturing PBMCs overnight with the peptide pool in the presence of brefeldin A (Sigma-Aldrich). T cells at a concentration of 1 × 106/mL were mixed with peptide-loaded or unmodified PBMCs or B-lymphoblastoid cell lines at an effector-stimulator cell ratio of 5:1. The cells were then stained for surface markers, fixed and permeabilized, and stained for intracellular IFN-γ, before acquisition and analysis. MHC-tetramer complexes were generated in the MSKCC Tetramer Core Facility. T cells were incubated with 25 μg/mL PE-labeled tetrameric complex on ice for 30 minutes, washed, and then stained with 10 μL of monoclonal anti-CD3 allophycocyanin, 10 μL of anti-CD8 peridin chlorophyll protein, and 10 μL of anti-CD62L FITC (BD Biosciences) for 20 minutes on ice. The stained cells were analyzed and quantitated with a FACSCalibur flow cytometer (BD Biosciences).
TREC analysis
Real-time quantitative PCR was performed using the TaqMan assay on cell lysates from purified CD4+ and CD8+ T cells according to Douek et al.28 CD4+ and CD8+ T cells were purified from freshly thawed PBMCs by Dynal CD4 or CD8 Positive Isolation Kit (Invitrogen), washed once with PBS before the pellets were frozen at −80°C. A total of 100 000 cells were lysed in 10 μL of 100 μg/mL proteinase K (Boehringer-Mannheim) in 10mM Tris, and heated for 1 hour at 56°C, and then for 10 minutes at 95°C. Real-time quantitative PCR was performed using the TaqMan assay on cell lysates by an ABI7500 system (Applied Biosystems). Multiplex quantitative PCR reactions were done in 20 μL with 1 unit of Platinum Taq DNA polymerase (Invitrogen), 2μM MgCl2, 200μM dNTPs (ABI), 500nM ROX (Stratagene), 500nM of each primer, 250nM probe for TCR-expressing circles (TRECs; Synthesis by Sigma Genosys) and primer and probe mix for RNase P (TaqMan RNase P Control Reagents kit [VIC], Applied Biosystems). PCR conditions were 50°C for 10 minutes, 95°C for 10 minutes, then 95°C for 15 seconds and 60°C for 1 minute for 45 cycles. The forward and reverse TREC primers were 5′-CACATCCCTTTCAACCATGCT-3′ and 5′-GCCAGCTGCAGGGTTTAGTG-3′, respectively, and the probe FAM-5′-ACACCTCTGGTTTTTGTAAAGGTGCCCACT-BHQ. A standard curve (101-5 TREC and RNase P copies/well) was plotted using a plasmid containing the TREC gene sequence (provided by D. C. Douek, National Institutes of Health, Bethesda, MD) and genomic DNA from human melanoma cell line SK-MEL-27. We normalized TREC values to cell content by concurrent measurement of the single-copy gene, RNase P. Samples were analyzed in duplicate, and results were averaged and normalized as TRECs/200 000 copies RNaseP, equaling TRECs/100 000 cells.
Combinatorial diversity analysis of T-cell repertoire
The T-cell repertoire diversity was measured using the Human ImmunTraCkeRβ test (ImmunID Technologies), a technique based on genomic DNA Multi-N-plex PCR.29 Genomic DNA was extracted from total PBMCs. Multi-N-plex PCR was performed using an upstream primer specific of all functional members of a given ΤΡβV family and a downstream primer specific of a given TRβJ segment. This assay allows the simultaneous detection of several V-J rearrangements in the same reaction and makes it possible to detect 276 different TRβV-TRβJ rearrangements covering 100% of the possible combinatorial rearrangements. The International ImMunoGeneTics information system (http://www.imgt.org) nomenclature was used for the designation of β-chain of human T-cell receptor (hTRβ) genes. Each V-J1, J2, J3, J4, and Jn product was separated as a function of its size on a 0.8% agarose gel, directly stained with SyberGreen I and quantified using a CCD camera equipped with BIO-1D (Vilber Lourmart). The Constel'ID Version 5.30 software (ImmunID Technologies) was used for further analytical studies, including generation of 3D repertoires illustrations. TCR diversity results are expressed as percentages of detected rearrangements among the 276 total possible combinatorial rearrangements.
Statistics
T-cell recovery was based on comparisons between changes in counts from day 21 or day 28, relative to baseline. Baseline counts are defined as the average of the week −1 and 0 measurements. A paired t test evaluated the average change from baseline, and ANOVA assessed any difference in the mean counts across dose levels. A generalized estimating equation was fit to historical data from a cohort of 185 patients who underwent TCD-HSCT to estimate the average weekly increase in CD4 and CD8 counts from 3 to 6 months after transplantation. Both models were fit on the log transform of the cell counts because of the skewness of the data. Significance was defined as P < .05 based on 2-sided tests. Statistical analyses were conducted using R Version 2.13.2 statistical software.
Results
CYT107 was not associated with significant toxicity
CYT107 is a fully glycosylated IL-7 produced in a CHO cell line. CYT107 was given subcutaneously weekly for 3 injections in successive cohorts of 3-6 patients in a standard phase 1 design (10, 20, and 30 μg/kg/dose). Twelve patients, including 6 men and 6 women, 27-67 years of age (median, 60 years) with hematologic malignancies were treated (Table 1). All patients underwent myeloablative cytoreduction that included antithymocyte globulin to prevent rejection, and a CD34-selected graft.30,31 The median day for starting CYT107 was 103 days (range, 60-244 days) after transplantation. Transient injection site reactions and low-grade fever were each noted in 2 patients. Three patients experienced grade 2 biopsy-proven hypersensitivity skin rash; patient 14-203 developed a rash after one injection and was removed from the study. The rash resolved without intervention. A grade 2 or higher rash was defined as a dose-limiting toxicity in the protocol, and the second dose cohort was expanded as a result. The other 2 patients developed a rash 8 days and 4 weeks after completing treatment, and both responded to a brief course of topical steroids. One patient developed grade 3 acute skin GVHD 11 weeks after treatment and was treated with systemic steroids. One patient had an incidental finding of splenomegaly on CT scan. Patient 14-301 developed an EBV-PTLD 10 weeks after treatment and responded to rituximab. No patients developed anti–IL-7 antibodies. With a median follow-up of 20.6 months, 9 patients remain alive. Two patients with high-risk acute myeloid leukemia died of relapse at 4 and 9 months after CYT107. Patient 14-203, who received one dose of CYT107, remained profoundly lymphopenic and died of progressive multifocal leukoencephalopathy 23 months after treatment.
Patient characteristics
| Dose level . | Patient no. . | Age, y . | Diagnosis . | Regimen . | Donor . | Day of CYT107 initiation after HSCT . | Baseline T-cell counts, /mm3* . | ||
|---|---|---|---|---|---|---|---|---|---|
| CD3 . | CD4 . | CD8 . | |||||||
| 10 μg/kg | 14-101 | 67 | AML | Bu/Mel/Flu | MRD | 96 | 526 | 219 | 299 |
| 14-102 | 55 | AML | TBI/Thio/Flu | MUD | 110 | 133 | 102 | 32 | |
| 14-103 | 59 | AML | Bu/Mel/Flu | MUD | 76 | 403 | 272 | 118 | |
| 20 μg/kg | 14-201 | 27 | AML | Bu/Mel/Flu | MRD | 244‡ | 233 | 124 | 101 |
| 14-203† | 67 | AML | Bu/Mel/Flu | MMUD | 222 | 35 | 30 | 17 | |
| 14-204 | 54 | AML | TBI/Thio/Cy | MRD | 73 | 69 | 20 | 55 | |
| 14-205 | 43 | AML | TBI/Thio/Cy | MUD | 208 | 89 | 69 | 24 | |
| 14-206 | 61 | MDS | Bu/Mel/Flu | MRD | 69 | 32 | 21 | 10 | |
| 14-207 | 63 | MDS | Bu/Mel/Flu | MRD | 61 | 13 | 5 | 9 | |
| 30 μg/kg | 14-301 | 39 | CML | TBI/Thio/Cy | MUD | 60 | 121 | 46 | 75 |
| 14-302 | 62 | AML | Bu/Mel/Flu | MRD | 109 | 262 | 262 | 10 | |
| 14-303 | 65 | AML | Bu/Mel/Flu | MRD | 110 | 0 | 0 | 0 | |
| Dose level . | Patient no. . | Age, y . | Diagnosis . | Regimen . | Donor . | Day of CYT107 initiation after HSCT . | Baseline T-cell counts, /mm3* . | ||
|---|---|---|---|---|---|---|---|---|---|
| CD3 . | CD4 . | CD8 . | |||||||
| 10 μg/kg | 14-101 | 67 | AML | Bu/Mel/Flu | MRD | 96 | 526 | 219 | 299 |
| 14-102 | 55 | AML | TBI/Thio/Flu | MUD | 110 | 133 | 102 | 32 | |
| 14-103 | 59 | AML | Bu/Mel/Flu | MUD | 76 | 403 | 272 | 118 | |
| 20 μg/kg | 14-201 | 27 | AML | Bu/Mel/Flu | MRD | 244‡ | 233 | 124 | 101 |
| 14-203† | 67 | AML | Bu/Mel/Flu | MMUD | 222 | 35 | 30 | 17 | |
| 14-204 | 54 | AML | TBI/Thio/Cy | MRD | 73 | 69 | 20 | 55 | |
| 14-205 | 43 | AML | TBI/Thio/Cy | MUD | 208 | 89 | 69 | 24 | |
| 14-206 | 61 | MDS | Bu/Mel/Flu | MRD | 69 | 32 | 21 | 10 | |
| 14-207 | 63 | MDS | Bu/Mel/Flu | MRD | 61 | 13 | 5 | 9 | |
| 30 μg/kg | 14-301 | 39 | CML | TBI/Thio/Cy | MUD | 60 | 121 | 46 | 75 |
| 14-302 | 62 | AML | Bu/Mel/Flu | MRD | 109 | 262 | 262 | 10 | |
| 14-303 | 65 | AML | Bu/Mel/Flu | MRD | 110 | 0 | 0 | 0 | |
AML indicates acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; Bu, busulfan; Mel, melphalan; Flu, fludarabine; TBI, total body irradiation; Thio, thiotepa; Cy, cyclophosphamide; MRD, matched related donor; MUD, matched unrelated donor; and MMUD, mismatched unrelated donor.
Baseline T-cell counts represent the mean of 2 consecutive tests before treatment, including 1 test performed immediately before the initial injection.
Patient 14-203 was removed from study after the first injection.
Patient 14-201 was treated with an approved protocol deviation starting at day 244 after HSCT because of poor immune recovery determined at 6 months after HSCT.
Pharmacokinetics
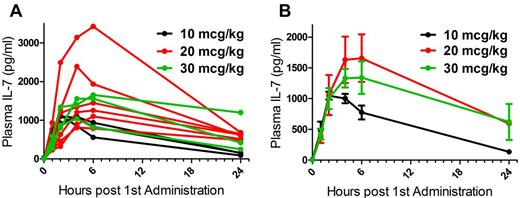
Pharmacokinetics were performed after the first and last CYT107 doses (Table 2; Figure 1). The Cmax ranged from 1048.5 to 1656.8 pg/mL. The global half-life was estimated at 9-35 hours.
Pharmacokinetics
| . | Dose level . | ||
|---|---|---|---|
| 10 μg/kg . | 20 μg/kg . | 30 μg/kg . | |
| Mean total daily dose, μg | 720 | 1353 | 1833 |
| Cmax, pg/mL | 1048.5 | 1656.8 | 1341 |
| Tmax, h | 2 | 6 | 6 |
| AUC total, pg/mL per min | 795 574 | 4 645 770* | 3 207 650 |
| t1/2, h | 8.7 | 40.2* | 34.6 |
| Clearance, mL/min | 905 | 259* | 571 |
| . | Dose level . | ||
|---|---|---|---|
| 10 μg/kg . | 20 μg/kg . | 30 μg/kg . | |
| Mean total daily dose, μg | 720 | 1353 | 1833 |
| Cmax, pg/mL | 1048.5 | 1656.8 | 1341 |
| Tmax, h | 2 | 6 | 6 |
| AUC total, pg/mL per min | 795 574 | 4 645 770* | 3 207 650 |
| t1/2, h | 8.7 | 40.2* | 34.6 |
| Clearance, mL/min | 905 | 259* | 571 |
In the 20-μg/kg cohort, PK parameters could only be calculated for 3 of 6 patients treated. IL-7 plasma concentrations at 24 hours were still elevated in 3 patients, and there was no available sample until day 14 to document IL-7 decrease.
Pharmacokinetic studies demonstrate rapid plasma clearance of IL-7 after injection of CYT107. (A) Individual patients at 3 dose levels. (B) Data are mean ± SEM for each dose level.
Pharmacokinetic studies demonstrate rapid plasma clearance of IL-7 after injection of CYT107. (A) Individual patients at 3 dose levels. (B) Data are mean ± SEM for each dose level.
CYT107 increases T-cell recovery after TCD-HSCT
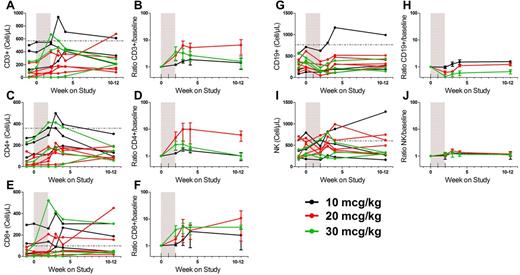
As expected early after TCD-HSCT, most patients were profoundly lymphopenic at baseline (Table 1). Mean CD3+, CD4+, and CD8+ counts were 170/mm3 (median, 121/mm3; range, 0-526/mm3), 102/mm3 (median, 69/mm3; range, 0-272/mm3), and 66/mm3 (median, 32/mm3; range, 0-299/mm3), respectively. After CYT107 treatment, there was a significant increase in CD3+ (average rise, 181/mm3 at day 21, P = .004, range −6 to 454/mm3, Figure 2A), CD4+ (average rise, 107.4/mm3 at day 21, P = .002; range, 0-279/mm3, Figure 2C) and CD8+ T cells (average rise, 66.9/mm3 at day 28, P = .05; range, −36 to 271/mm3, Figure 2E) compared with baseline values in 11 evaluable patients. These increases represented mean rises of 4.3-fold (range, 0.9- to 13.5-fold), 6.1-fold (range, 1.1- to 35-fold), and 4.3-fold (range, 0.7- to 11-fold) in CD3+, CD4+ and CD8+ counts, respectively (Figure 2B,D,F). No significant dose effect was observed. There was a trend toward a decrease in circulating B cells (average decrease, −68.4/mm3 at day 21, P = .06; range, −205 to 172/mm3, Figure 2G-H). No effect was observed on circulating NK cells (Figure 2I-J).
CYT107 induces increases in T cells in the peripheral blood but does not affect NK cells or B cells. (A) Absolute CD3 counts. (B) Ratio CD3 count/baseline. (C) Absolute CD4 counts. (D) Ratio CD4 count/baseline. (E) Absolute CD8 counts. (F) Ratio CD8 count/baseline. (G) Absolute B-cell counts. (H) Ratio B-cell count/baseline. (I) Absolute NK-cell counts. (J) Ratio NK count/baseline. Absolute counts are shown for individual patients. The ratios (mean ± SEM) are shown for each cohort and are calculated using as a baseline value the mean of the pretreatment and day 0 values. The shaded area represents the CYT107 treatment interval (injections were given once a week as described in “Results”). (A,C,E,G,I) The horizontal line indicates the lower limit of normal.
CYT107 induces increases in T cells in the peripheral blood but does not affect NK cells or B cells. (A) Absolute CD3 counts. (B) Ratio CD3 count/baseline. (C) Absolute CD4 counts. (D) Ratio CD4 count/baseline. (E) Absolute CD8 counts. (F) Ratio CD8 count/baseline. (G) Absolute B-cell counts. (H) Ratio B-cell count/baseline. (I) Absolute NK-cell counts. (J) Ratio NK count/baseline. Absolute counts are shown for individual patients. The ratios (mean ± SEM) are shown for each cohort and are calculated using as a baseline value the mean of the pretreatment and day 0 values. The shaded area represents the CYT107 treatment interval (injections were given once a week as described in “Results”). (A,C,E,G,I) The horizontal line indicates the lower limit of normal.
To further assess the impact of CYT107 on immune recovery, we studied a cohort of 185 patients with hematologic malignancies who underwent TCD-HSCT using the same transplant regimens but who did not receive any further posttransplantation interventions (J.D.G., Zheng J, Barker JN, Boulad F, Castro-Malaspina HR, Hsu KC, Jakubowski AA, Kernan NA, O'Reilly RJ, Papadopoulos EB, Prockop S, Scaradavou A, van den Brink MRM, Young JW, Heller M, Perales MA, Early immune recovery predicts overall and disease-free survival after allogeneic hematopoietic stem cell transplantation, manuscript in preparation). The median age for the control cohort was 54.0 years (range, 18.5-72.9 years). In the historical data, the estimated weekly increase in CD4 and CD8 between months 3 and 6 after transplantation was 1.04 per week (95% CI, 1.02-1.07) and 1.05 per week (95% CI, 1.03-1.08), respectively. Although these increases were less than what was observed for patients treated with CYT107, a randomized study will be required to confirm these preliminary results.
Furthermore, we reviewed viral infections in patients on the study to determine whether any of the rises in T cells could be attributed to viral-specific responses. There were 3 cases of CMV viremia. Patient 14-102 completed maintenance valganciclovir one month before starting CYT107 and had no further viremia. Patient 14-206 had a significant increase in CMV-specific responses and is described in greater detail below on functional responses. The final patient who reactivated CMV was patient 14-301 who never required treatment. The CMV-PCR was 1870 copies/mL on day −7, 2324 copies/mL on day −5, 1554 copies/mL on day 0, 1202 copies/mL on day 7, and negative thereafter. This patient did have CD4 and CD8 increases in response to CYT107 but no significant rise in the prior week. Unfortunately, we were not able to study CMV-specific responses in this particular patient. Overall, the incidence of viral infections in this population was low, and we cannot attribute all responses in CD4 and CD8 counts to viral responses.
Analysis of T-cell subsets demonstrates an increase in effector memory T cells
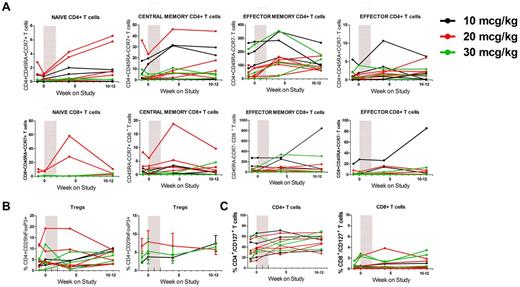
To further delineate the effects on T-cell recovery, we examined specific CD4+ and CD8+ subsets.32-36 For both CD4+ and CD8+ T cells, the main increase in absolute numbers was in the effector memory subset, which represented the largest T-cell subset at baseline (89.8% ± 7.3% and 85.7% ± 11.1% of CD4+ and CD8+ T cells at baseline, respectively; Figure 3A). More modest increases were observed in other subsets, including naive T cells in a subgroup of patients. In CD8+ T cells in particular, the increase in naive cells was more pronounced in 2 patients in the 20-μg/kg cohort (up to 38% absolute increase). There appeared to be no change in the frequency of regulatory T cells (Tregs, mean frequency 2.2%-7.9% throughout the study; Figure 3B). No effect was seen on the % CD4+ T cells expressing inducible costimulator, a costimulatory molecule associated with tumor rejection and up-regulated on CD4+ T cells after ipilimumab (not shown).37,38
CYT107 induces the expansion of effector memory CD4+ and CD8+ T cells but no changes in Treg or CD127 expression. (A) CYT107 predominantly induces an expansion of effector memory T cells with an increase in naive CD4+ and CD8+ T cells in a minority of patients. Each panel represents absolute values of T-cell subsets of CD4+ or CD8+ T cells using CD45RA and CCR7 expression to distinguish naive (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), and effector (CD45RA+CCR7−) T cells. Data for individual patients are shown. (B) CYT107 administration does not affect the relative frequency of Tregs, defined as CD4+CD25hiFoxP3+ T cells. Left: Absolute Treg counts for individual patients. Right: Mean ratio Treg count/baseline ± SEM for each cohort. (C) CYT107 administration does not affect long-term expression of CD127 on CD4+ or CD8+ T cells (data for individual patients shown).
CYT107 induces the expansion of effector memory CD4+ and CD8+ T cells but no changes in Treg or CD127 expression. (A) CYT107 predominantly induces an expansion of effector memory T cells with an increase in naive CD4+ and CD8+ T cells in a minority of patients. Each panel represents absolute values of T-cell subsets of CD4+ or CD8+ T cells using CD45RA and CCR7 expression to distinguish naive (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), and effector (CD45RA+CCR7−) T cells. Data for individual patients are shown. (B) CYT107 administration does not affect the relative frequency of Tregs, defined as CD4+CD25hiFoxP3+ T cells. Left: Absolute Treg counts for individual patients. Right: Mean ratio Treg count/baseline ± SEM for each cohort. (C) CYT107 administration does not affect long-term expression of CD127 on CD4+ or CD8+ T cells (data for individual patients shown).
We next assessed the expression of IL-7 α-receptor (CD127, Figure 3C). The baseline median expression of CD127 was 31.5% (range, 12.3%-62.8%) and 0.5% (range, 0.1%-2.2%) on CD4+ and CD8+ T cells, respectively. CYT107 did not cause long-term down-regulation of CD127. In most patients, CYT107 did not affect Ki-67 expression, a measure of T-cell proliferation,39 at the time points studied (not shown). In the 4 patients where changes were observed, no distinct pattern was identified. Although there was no effect on Bcl-2 expression in CD8+ T cells, 3 patients had increased Bcl-2 expression in CD4+ T cells (not shown).
No increase in TRECs was observed after CYT107
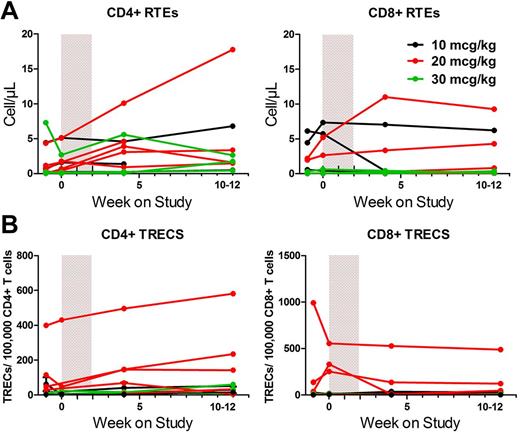
We then analyzed thymic output by assessing recent thymic emigrants (RTEs), identified by the CD4+CD45RA+CD31+CD62LbrightCD95dim and CD8+CD103+CD62LbrightCD95dim phenotypes,33 and TRECs. Patients had very low numbers of RTEs, and CYT107 administration did not affect their frequency, except in 2 of the youngest patients on the study where it increased RTEs (patients 14-201 and 14-205; Figure 4A). The baseline frequency of TRECs was variable among patients, with the highest frequency observed in patient 14-201, who was 27 years old and the youngest patient in the study (Figure 4B). The ages of the other 2 patients with detectable TRECs were 43 years (14-205) and 54 years (14-204). Similar to RTEs, no apparent effect was seen on TREC frequency.
CYT107 did not have a significant effect on thymic output in most patients. (A) Absolute CD4+ and CD8+ RTE counts, identified by the CD4+CD45RA+CD31+CD62Lbright CD95dim and CD8+CD103+CD62LbrightCD95dim phenotypes for individual patients. (B) CD4+ and CD8+ TRECs are shown for individual patients in each cohort.
CYT107 did not have a significant effect on thymic output in most patients. (A) Absolute CD4+ and CD8+ RTE counts, identified by the CD4+CD45RA+CD31+CD62Lbright CD95dim and CD8+CD103+CD62LbrightCD95dim phenotypes for individual patients. (B) CD4+ and CD8+ TRECs are shown for individual patients in each cohort.
CYT107 induces functional T-cell responses
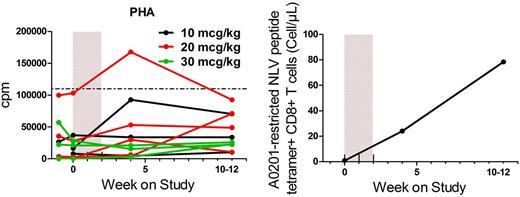
CYT107 induced increased responses to PHA (Figure 5A) and mixed pooled lymphocytes in 7 and 9 of 11 evaluable patients (not shown), respectively. No changes in responses to recall or viral antigens were observed (not shown). However, in 4 CMV-seropositive patients evaluated, increased IFN-γ production to CMV overlapping peptide pools was observed in 3 patients (not shown). In addition, patient 14-206, who was receiving valganciclovir for CMV viremia at the time of initiation of CYT107, had a rapid and notable increase in the number of CMV-specific CD8+ T cells, detected by tetramer (Figure 5B) and IFN-γ production (not shown). The low-level viremia (maximal antigenemia 13+ cells/slide) preceded the treatment with CYT107 by 4 weeks, and the rise in CMV-specific CD8 T cells detected by tetramer was coincident with administration of IL-7. The CMV antigenemia was undetectable on day 0, and other than a small rise on day 7 (5+ cells/slide), remained negative thereafter.
Administration of CYT107 enhances functional T-cell responses. (A) Reponses to PHA for individual patients. The horizontal line indicates the lower limit of normal. (B) Rapid increase in CMV-specific responses in patient 14-206 determined by A0201-restricted pp65 immunodominant peptide NLVPMVATV (NLV) tetramer.
Administration of CYT107 enhances functional T-cell responses. (A) Reponses to PHA for individual patients. The horizontal line indicates the lower limit of normal. (B) Rapid increase in CMV-specific responses in patient 14-206 determined by A0201-restricted pp65 immunodominant peptide NLVPMVATV (NLV) tetramer.
Analysis of hTRβ immune repertoire
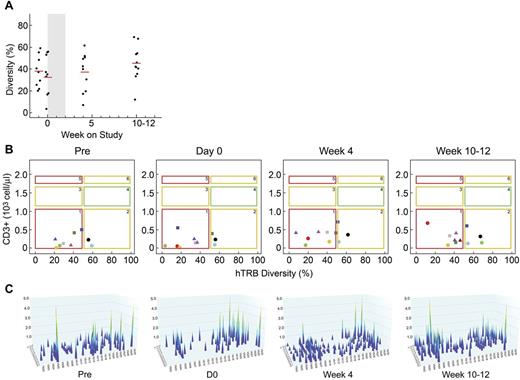
To further characterize the observed T-cell recovery, we analyzed the T-cell repertoire using a genomic DNA Multi-N-plex PCR technique (Figure 6). The average hTRβ diversity in healthy persons is 67% ± 11% and diversity < 50% is considered low (J. F. Mouret, ImmunID Technologies, written personal communication, April 2012). In the present study, the mean diversity at baseline for all patients was 34% (range, 14.7%-57.2%, pooled data for days −7 and 0; Figure 6A). Only 2 patients (14-201 and 14-205) had a diversity > 50%. These are the same younger patients in which an increase in naive T cells and RTEs, as well as the presence of TRECs, was observed. At day 28, 7 of 10 evaluable patients had increased hTRβ diversity compared with baseline, whereas 3 patients had a decrease in diversity (Figure 6A). At the end of the study, 7 of 11 patients had an increase in diversity, including 4 patients with a diversity > 50%. Patient 14-103 with the largest drop in hTRβ diversity at the end of the study also had the largest drop in T-cell counts at that time.
Administration of CYT107 enhances TCR diversity. (A) Evolution of the mean combinatorial diversity (hTRβ) over time. Individual patients and the mean diversity are shown. The shaded area on each graph represents the CYT107 treatment interval. For patient 14-303, only the last study time point was available for analysis because of profound lymphopenia before that sample. (B) Relationship over time between T-cell counts and hTRβ (NDL representation). Most patients shift from NDL1 (low CD3/low diversity) toward NDL2 (low CD3/normal diversity) after treatment with CYT107. Individual patients are shown. Squares, circles, and triangles represent patients in the 10-, 20-, and 30-μg/kg cohorts, respectively. (C) The image represents a 3D graph of immune combinatorial diversity at each studied time point (pre, D0, D28, D77) after treatment with CYT107 in representative patient 14-207. Each peak represents the rearrangement between a V gene family and a J segment. V families are represented on the x-axis. J segments are represented on the y-axis. The intensity of these rearrangements is represented on the z-axis, varying between 0 and 5.
Administration of CYT107 enhances TCR diversity. (A) Evolution of the mean combinatorial diversity (hTRβ) over time. Individual patients and the mean diversity are shown. The shaded area on each graph represents the CYT107 treatment interval. For patient 14-303, only the last study time point was available for analysis because of profound lymphopenia before that sample. (B) Relationship over time between T-cell counts and hTRβ (NDL representation). Most patients shift from NDL1 (low CD3/low diversity) toward NDL2 (low CD3/normal diversity) after treatment with CYT107. Individual patients are shown. Squares, circles, and triangles represent patients in the 10-, 20-, and 30-μg/kg cohorts, respectively. (C) The image represents a 3D graph of immune combinatorial diversity at each studied time point (pre, D0, D28, D77) after treatment with CYT107 in representative patient 14-207. Each peak represents the rearrangement between a V gene family and a J segment. V families are represented on the x-axis. J segments are represented on the y-axis. The intensity of these rearrangements is represented on the z-axis, varying between 0 and 5.
We next analyzed the relationship over time between CD3+ T-cell counts and hTRβ diversity using a 2-dimensional representation termed number and diversity of lymphocytes (NDL, Figure 6B).40 This model identifies 6 potential combinations of T-cell counts (< normal, normal, and > normal) and hTRβ diversity (< or > 50%). As an example, individuals with a normal immune system have normal T-cell counts and a diversity > 50% (NDL4). We observed a shift from NDL1 (low CD3/low diversity) toward NDL2 (low CD3/normal diversity) in 10 of 11 patients after CYT107. Despite this shift, however, 7 patients remained lymphopenic with low diversity at the end of study, and potentially at risk for opportunistic infections.
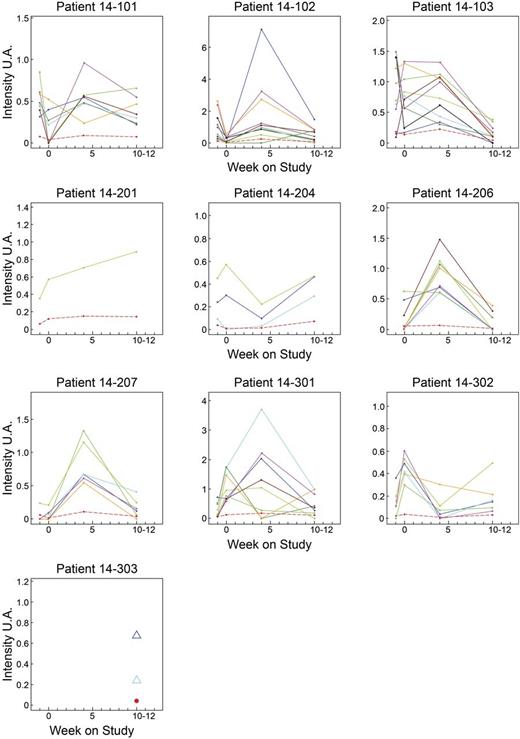
Finally, we analyzed major hTRβ-VJ rearrangements, defined as those representing > 2% of the overall repertoire (based on intensity levels, Figure 7). Major rearrangements were detected in 10 of 11 patients, and the median number was 6 (range, 0-10). In 7 patients, the majority of the rearrangements were detected at day 28. In 2 patients (14-204 and 14-302), the major rearrangements were detected at day 0 and were subsequently down-regulated.
Evolution over time of the major hTRB VJ rearrangements identified per patient. Only patient 14-205 has no major rearrangement detected. The sample at D-7 was not available for patient 14-206, and for patient 14-303 only data for D77 were available. Each color represents a major hTRβ VJ rearrangement. The red dotted line indicates the average for each patient.
Evolution over time of the major hTRB VJ rearrangements identified per patient. Only patient 14-205 has no major rearrangement detected. The sample at D-7 was not available for patient 14-206, and for patient 14-303 only data for D77 were available. Each color represents a major hTRβ VJ rearrangement. The red dotted line indicates the average for each patient.
Discussion
To enhance posttransplantation T-cell recovery, we performed a phase 1 study of r-hIL-7 (CYT107) in recipients of a TCD allo-HSCT and demonstrated that, consistent with prior studies of r-hIL-7, the drug was safe and well tolerated.20-25 There was one case of EBV-PTLD that responded to rituximab. We have previously reported that the incidence of EBV-PTLD in recipients of TCD-HSCT is 10%.1 Therefore, we cannot attribute the case we observed to treatment with r-hIL-7. Similarly, the 2 cases of acute myeloid leukemia relapse are consistent with the predicted incidence of recurrence after allo-HSCT because of their high-risk features. Unlike prior studies with Escherichia coli produced r-hIL-7 (CYT99007), we did not detect antibodies to IL-7. However, patients in solid tumor studies not only received nonglycosylated IL-7 but also had a more robust immune system.20
A potential concern of IL-7 in allo-HSCT patients is GVHD. In preclinical models, short courses of IL-7 did not induce GVHD in TCD-BMT or aggravate the development of GVHD after adding donor T cells.15,18,41 In contrast, IL-7 lowered the threshold dose of T cells required to induce lethal GVHD when given for a longer course at high dose after TCD-BMT admixed with increasing T-cell doses.42 Only one patient in the present study developed acute skin GVHD after IL-7. Although we cannot exclude a contribution of IL-7, this low incidence of GVHD is consistent with our prior experience in TCD-HSCT.30,31 Three additional patients developed a skin rash, including the patient who was removed from study after a single injection. Although the pathologic diagnosis of GVHD can be challenging, the biopsy findings in all 3 were considered most consistent with hypersensitivity reactions and unlikely to represent GVHD. Furthermore, none of the patients had any other signs or findings suggestive of GVHD either at the time of the rash or subsequently in follow-up. Although IL-7 does not appear to increase GVHD after a TCD-HSCT, the capacity of IL-7 to cause or worsen GVHD in a conventional allo-HSCT remains uncertain and will require further investigation.
Overall, our immune recovery results are consistent with other phase 1 trials of r-hIL-7 in patients with solid tumors or HIV infection that demonstrated increases in CD4+ and CD8+ T cells.20-23 The prior studies reported an expansion of RTEs, naive and central memory, but not effector T cells, and a relative decrease in Tregs.20-23 In contrast, although we observed some increases in naive T cells and RTEs in a subset of patients, the main effect of IL-7 was an expansion of effector memory T cells, the predominant subset identified in our patients. These differences in results are probably explained by the fact that the median age of the patients treated on the present study was older than patients treated on the earlier studies of IL-7. For example, in the HIV studies, the median ages were 44 and 46 years.22,23 Furthermore, allo-HSCT patients are profoundly immunocompromised,1,7 and the baseline distribution of T-cell subsets is clearly different in patients early after TCD allo-HSCT compared with other patients who have received IL-7. Interestingly, recent studies have shown that naive human T cells can proliferate without taking on a memory phenotype as in mice.43
IL-7 has been implicated in the transition of effector to memory cells, and CD127 expression is thought to identify memory cell precursors.44,45 We found that CD127 expression in CD4+ and particularly CD8+ T cells was lower than that reported in healthy persons. Nevertheless, IL-7 induced expansions of both cell populations, with the larger expansion in CD4+ T cells probably the result of higher CD127 expression. Low expression of CD127 on CD8+ T cells has also been observed in HIV patients, where it correlated with an expansion of CD8+CD127− effector-like T cells associated with disease progression.46
Some of the other differences we observed compared with prior studies of IL-7 are probably related to the time points analyzed (day 28 and beyond day 70). In solid tumor or HIV patients treated with IL-7, the peak down-regulation in CD127 and up-regulation in Ki-67 was observed in the first 2 weeks and had returned to baseline by 2-3 weeks.21-23 We did, however, observe increased Bcl-2 expression in CD4+ T cells in some patients, consistent with the finding that IL-7 inhibits apoptosis in mature T lymphocytes through up-regulation of Bcl-2 family molecules.47
Importantly, we not only saw quantitative increases in T cells after a short course of IL-7, but also demonstrated an increase in functional T cells, including viral-specific T cells that recognize CMV, one of the most frequent infections after allo-HSCT. Improved responses to mitogens have previously been reported in cancer or HIV patients,21,22 and increases in HIV and CMV-specific CD4+ T cells were noted in HIV patients.22 The findings in this study and other studies of r-hIL-7 have potentially important bearing on the management of patients after allo-HSCT because delays in immune reconstitution and associated infections play a significant role in morbidity and mortality. Successful treatment with IL-7 of progressive multifocal leukoencephalopathy was recently reported in a patient with idiopathic CD4+ lymphocytopenia.48
Lymphopenia combined with low TCR diversity has been defined as divpenia and correlates with decreased overall survival in patients with metastatic breast cancer.40 The patients in the current study were profoundly lymphopenic and had low TCR diversity. Importantly, the increase in functional T cells we observe also correlates with our finding that IL-7 induces an increase in T-cell diversity. These results are consistent with a broadening of the TCR repertoire in cancer patients treated with IL-7 by traditional spectratyping analysis.21 The majority of major hTRβ VJ rearrangements were also detected at day 28. This may indicate that the increase in T cells is because of 2 main mechanisms: an immediate effect on preexisting T cells that proliferate in response to CYT107, and a subsequent effect through renewal of peripheral T cells, possibly through a thymopoietic effect, which is detected by increase of diversity that can take longer to occur.
This is the first study to demonstrate both quantitative and qualitative improvements in T-cell recovery after allo-HSCT without donor leukocyte infusion. Although the number of patients is too small to draw conclusions regarding an impact of IL-7 on risk of infection and relapse, there are significant data correlating immune recovery with those important clinical outcomes. We therefore hypothesize that IL-7 can decrease infections and relapse by improving immune recovery in allo-HSCT recipients. Future larger randomized studies, with potential redosing of IL-7 dictated by T-cell recovery,49 will be needed to confirm our initial results and address the clinical benefits of IL-7 in this high-risk patient population.
There is an Inside Blood commentary on this article in this issue.
Presented in part at the American Society of Hematology Meeting on December 6, 2010 (Orlando, FL), BMT Tandem Meetings on February 17, 2011 (Honolulu, HI), and the European Group for Blood and Marrow Transplantation Meeting on April 5, 2011 (Paris, France).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported in part by the National Institutes of Health (P01 CA23766; R01-HL069929, R01-CA107096, and R01-AI080455, M.R.M.v.d.B.) and by the US Department of Defense (US AMRAA Award W81XWH-09-1-0294, M.R.M.v.d.B.), the Radiation Effects Research Foundation (RERF-NIAID, M.R.M.v.d.B.), the Translational and Integrative Medicine Research Fund of Memorial Sloan-Kettering Cancer Center (M.-A.P.), the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr William H. Goodwin and Mrs Alice Goodwin (M.-A.P. and M.R.M.v.d.B.), Cycle for Survival (M.-A.P.), the New York Community Trust (M.-A.P.), When Everyone Survives (M.-A.P.), the Lymphoma Foundation (M.R.M.v.d.B.), the Leukemia & Lymphoma Society (M.-A.P. and M.R.M.v.d.B.), Alex's Lemonade Stand (M.R.M.v.d.B.), the Geoffrey Beene Cancer Research Center at Memorial Sloan-Kettering Cancer Center (M.R.M.v.d.B.), and the Peter Solomon Fund (M.R.M.v.d.B.). The study was sponsored by Cytheris Inc, subsidiary of Cytheris S.A.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.-A.P. designed and performed research, analyzed data, enrolled patients in the study, and wrote the manuscript; M.R.M.v.d.B. designed and performed research, analyzed data, and wrote the manuscript; J.D.G., B.Z., H.G., C.L., T.R., and J.D.W. performed research, analyzed data, and wrote the manuscript; J.Y. and G.K. designed and performed research and wrote the manuscript; E.B.P., J.W.Y., and A.A.J. enrolled patients in the study and wrote the manuscript; L.L. and S.M.D. analyzed data and wrote the manuscript; and T.C. and M.M. designed research and wrote the manuscript.
Conflict-of-interest disclosure: M.M. was CEO and shareholder of Cytheris SA; T.C. is an employee and shareholder of Cytheris SA; and M.-A.P. and M.R.M.v.d.B. received research support for the study. The remaining authors declare no competing financial interests.
Correspondence: Miguel-Angel Perales, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 298, New York, NY 10065; e-mail: peralesm@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal