Abstract
Natural killer (NK) cells become activated during viral infection in response to cytokines or to engagement of NK cell activating receptors. However, the identity of cells sensing viral particles and mediating NK cell activation has not been defined. Here, we show that local administration of a modified vaccinia virus Ankara vaccine in mice results in the accumulation of NK cells in the subcapsular area of the draining lymph node and their activation, a process that is strictly dependent on type I IFN signaling. NK cells located in the subcapsular area exhibited reduced motility and were found associated with CD169+-positive subcapsular sinus (SCS) macrophages and collagen fibers. Moreover, depletion of SCS macrophages using clodronate liposomes abolished NK cell accumulation and activation. Our results identify SCS macrophages as primary mediators of NK cell activation in response to lymph-borne viral particles suggesting that they act as early sensors of local infection or delivery of viral-based vaccines.
Introduction
Microbial infections often promote natural killer (NK) cell activation or priming, a process mediated, at least in part, by several cytokines including IL-12, IL-18, IL-15, and type I IFNs.1 Hallmarks of primed or activated NK cell include up-regulation of activation markers, increase in intracellular granzyme B content, production of IFN-γ, and rapid cytotoxic activity. Most features of NK cell priming are also observed on in vivo administration of TLR agonists,2 such as poly I:C, an analog of dsRNA, that engages TLR3 and MDA-5.3 It was previously shown that both dendritic cells (DCs) and stromal cells respond to poly I:C to promote NK cell activation.4 Moreover, type I IFNs are critical during this process and act both directly on NK cells and indirectly to promote IL-15 transpresentation on DCs.5-9 Recently, we used mice that specifically expressed the green fluorescent protein (GFP) and yellow fluorescent protein (YFP) in NK cells and DCs, respectively, to visualize NK cell priming on IV administration of poly I:C. We found that NK cells become activated while maintaining a migratory behavior, in the absence of long-lived contacts with DCs.10
Although poly I:C administration mimics certain aspects of systemic viral infection, the sequence of events mediating NK cell activation in response to lymph-borne viral particles has not been identified. This is particularly relevant because viruses delivered locally (through insect bites for example) are expected to reach the lymph,11 and as the local delivery of viral-based vector is being used for vaccination purposes.12 To address this issue, we analyzed and imaged by two-photon microscopy NK cell activation in response to subcutaneous delivery of recombinant modified vaccinia virus Ankara (MVA), an attractive viral vector that is being considered for vaccination against cancer and infectious diseases12,13 We used a clinical grade MVA preparation, termed MVA-HIV, developed by the ANRS, (the French National Agency against AIDS and Hepatitis), and expressing HIV Gag, Pol, and Nef antigens. We previously reported that in culture, this vector efficiently infects primary human macrophages, dendritic cells (DCs), and epithelial and muscle cells.14 In addition, exposure of DCs or macrophages to MVA cell-free particles or to MVA-infected cells are known to induce type I IFN production.14,15
We report here that upon injection of MVA in mice, NK cells rapidly accumulate and decelerate in subcapsular areas of the draining lymph node and become activated in a type I IFN-dependent manner. Moreover, depletion of SCS macrophages before MVA injection abolished NK cell accumulation and activation. These data strongly suggest that SCS macrophages play a pivotal role during NK cell activation by lymph-borne viral particles.
Methods
Mice
C57Bl/6 mice were purchased from Charles River France. NCR1GFP/+,16 CXCR3−/−, CCR5−/−, IL-15−/−, and IFNAR−/− mice were bred in our animal facility under specific pathogen-free conditions. Animal experiments were performed in accordance to Institut Pasteur guidelines for animal care and use.
Injections
Recombinant MVA expressing an HIV polyprotein (containing full-length Gag, fused to 3 Pol and 2 Nef fragments) was described previously,14 and was provided by the ANRS. Mice were injected in the footpad with 5 × 106 pfu of MVA or 50 μg poly I:C (Invivogen). Clodronate was encapsulated in liposomes as described earlier.17 For macrophage depletion, clodronate or control (PBS) liposomes were injected in the footpad 6 days before MVA injection. For in vivo labeling of CD169-positive cells, FITC-conjugated anti-CD169 Fab fragment was injected in the footpad of recipient mice 1 hour before imaging.
Flow cytometry and ELISA
Cell-surface staining performed using the following antibodies in the presence of blocking anti-CD16/32 (eBioscience): eFluor 450-conjugated anti-CD3, PE-Cy7–conjugated anti-NK.1.1, APC-Cy7–conjugated anti-CD11b (eBioscience), FITC-conjugated anti-CD69 (Biolegend), and FITC-conjugated anti-CD169 (AbD Serotec). Intracellular staining was performed using the Cytofix/Cytoperm kit (BD Bioscience) using APC-conjugated antigranzyme B (Biolegend) and PE-conjugated anti–IFN-γ (BD Bioscience). Analysis was performed on a FACS Canto II (BD Bioscience). Data were analyzed using the FlowJo software (version 9.1, Tree Star). For measurement of IFN-α levels, intact lymph nodes were cultured at 37°C for 16 hours. Cytokine production in the supernatant was measured using a mouse IFN-α platinum ELISA kit (eBioscience) following the manufacturer's instructions.
Two-photon imaging
Two-photon imaging of intact popliteal lymph node was performed as previously described18 on an upright microscope (DM6000B, Leica Microsystems) with a 20×/0.95 NA water-dipping objective (Olympus). Excitation was provided by a Chameleon Ultra Ti:Sapphire (Coherent) tuned at 960 nm. Datasets were processed and analyzed using Imaris (Bitplane) and the custom designed DISCit Version 1.0 software21 for conversion into FCS files. Imaging data were then displayed as dot-plot profiles and histograms using FlowJo Version 8.8.7 software.
Statistical analyses
All data are represented as mean ± SEM. Statistical analyses were performed using Prism Version 5 software (GraphPad) and unpaired Student t, Mann-Whitney, or 1-way ANOVA test. P values lower than .05 were considered significant.
Results
Local delivery of MVA induces NK cell recruitment and activation in the draining lymph node
To study how lymph node NK cells sense lymph-borne viral particles, we injected C57BL/6 mice in the footpad with a recombinant MVA virus (5 × 106 pfu), a vaccine candidate against HIV developed by the ANRS (hereafter referred to as MVA). We found that 16 hours after MVA administration, NK cells in the draining lymph node increased by 10-fold in absolute numbers and by 3-fold in percentage (Figure 1A). To better define the cues that promoted NK cell accumulation in the draining lymph node, we analyzed the response of mice deficient for either CXCR3 or CCR5, 2 chemokine receptors that were previously shown to contribute to NK cell trafficking. Interestingly, CXCR3−/− but not CCR5−/− recipients displayed a dampened NK cell accumulation compared with WT animals (Figure 1B-C). Altogether our results indicate that MVA induces a sizeable increase in NK cell numbers in the draining lymph node that was partly dependent on the CXCR3 receptor.
Local administration of MVA induces NK cell recruitment in the draining lymph node. WT (C57BL/6), CXCR3−/− or CCR5−/− mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. After 16 hours, cells from the draining popliteal lymph node were analyzed by flow cytometry. (A) NK cell accumulation after MVA injection. Profiles are gated on CD3− cells. Numbers correspond to percentage of cells falling in the indicated gate. (B) The absolute numbers of NK cells in the draining lymph node is graphed for WT, CXCR3−/−, or CCR5−/− mice. (C) The percentage of NK cells (NK1.1+CD3−) is graphed for WT, CXCR3−/−, or CCR5−/− mice. Data are representative of 3 independent experiments (**P < .01).
Local administration of MVA induces NK cell recruitment in the draining lymph node. WT (C57BL/6), CXCR3−/− or CCR5−/− mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. After 16 hours, cells from the draining popliteal lymph node were analyzed by flow cytometry. (A) NK cell accumulation after MVA injection. Profiles are gated on CD3− cells. Numbers correspond to percentage of cells falling in the indicated gate. (B) The absolute numbers of NK cells in the draining lymph node is graphed for WT, CXCR3−/−, or CCR5−/− mice. (C) The percentage of NK cells (NK1.1+CD3−) is graphed for WT, CXCR3−/−, or CCR5−/− mice. Data are representative of 3 independent experiments (**P < .01).
Next, we assessed whether NK cells accumulating in the draining lymph nodes became activated on MVA injection. We found that, at 16 hours, lymph node NK cells up-regulated the CD69 activation marker and displayed a strong increase in intracellular granzyme B content (Figure 2A). In addition, approximately 20% of NK cells residing in the draining lymph node produced IFN-γ. When we examined the phenotype of NK cells in the lymph nodes with respect to CD27 and CD11b expression, we found that MVA resulted in the preferential accumulation of CD11b+CD27+ NK cells but activation of all NK cell subsets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition, a kinetic analysis revealed that some of the hallmarks of NK cell activation were detected up to day 3 (supplemental Figure 2). Because type I IFNs play an important role during NK cell activation induced by TLR agonists or viruses,2,5 we analyzed how mice lacking the IFNAR1 (referred to as IFNAR−/− mice) responded to MVA. As shown in Figure 2A and B, MVA-induced NK cell activation was virtually abolished in IFNAR−/− mice, with little to no increase in granzyme B content, CD69 levels, or IFN-γ production. Thus, local administration of MVA triggers potent NK cell activation that is critically dependent on type I IFN signaling.
IFNAR signaling is required for MVA-induced NK cell activation. WT or IFNAR−/− mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. After 16 hours, NK cells in the draining lymph node were analyzed for CD69 and for intracellular granzyme B and IFN-γ. (A) Representative FACS profiles showing granzyme B, CD69, and IFN-γ staining in NK cells. Data are gated on NK1.1+CD3− cells. (B) The percentages of granzyme Bhigh, CD69+ and IFN-γ+ NK cells (NK1.1+CD3− cells) are compiled for WT and IFNAR−/− recipients treated or not with MVA. Each dot represents an individual mouse. Data are compiled from 2 to 3 independent experiments (***P < .001).
IFNAR signaling is required for MVA-induced NK cell activation. WT or IFNAR−/− mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. After 16 hours, NK cells in the draining lymph node were analyzed for CD69 and for intracellular granzyme B and IFN-γ. (A) Representative FACS profiles showing granzyme B, CD69, and IFN-γ staining in NK cells. Data are gated on NK1.1+CD3− cells. (B) The percentages of granzyme Bhigh, CD69+ and IFN-γ+ NK cells (NK1.1+CD3− cells) are compiled for WT and IFNAR−/− recipients treated or not with MVA. Each dot represents an individual mouse. Data are compiled from 2 to 3 independent experiments (***P < .001).
MVA induces accumulation of NK cells in the subcapsular area of the lymph node
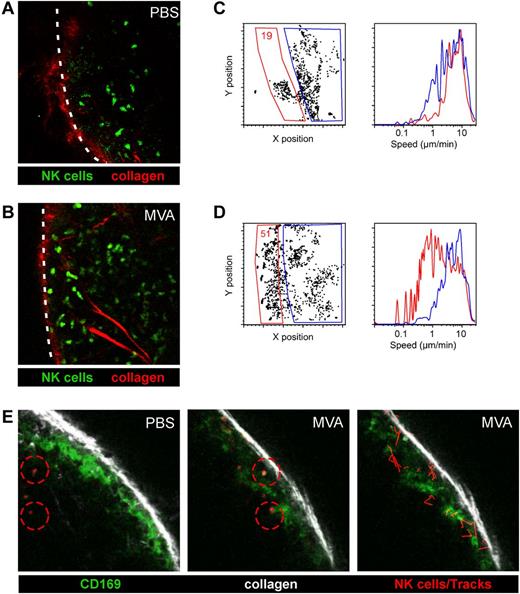
To assess how local delivery of virus particles may influence NK cell behavior, we analyzed NK cell dynamics in lymph nodes. We injected PBS or MVA in the footpad of NCR1GFP/+ mice, in which all NK cells are specifically fluorescently labeled,16 and performed two-photon imaging on intact popliteal lymph nodes. In control mice, NK cells were motile and mostly enriched in the interfollicular/outer T-cell zone of the lymph nodes as previously described.10,19,20 Interestingly, a large fraction of NK cells redistributed to the subcapsular area in MVA treated mice (Figure 3A-B, supplemental Video 1). To quantify this phenomenon, we took advantage of DISC, a methodology that we recently developed in which imaging datasets are processed, converted into an FCS file, and subjected to multiparametric analysis using a flow cytometry software.21 Through gating strategies, DISC allowed us to quantify NK cell distribution and velocity in distinct locations of the lymph node. As shown in Figure 3C and D, there was a 3-fold increase in the percentage of NK cells present in the subcapsular areas of the lymph node in MVA treated mice. Moreover, these NK cells displayed a strongly diminished motility compared with NK cells present in deeper region of the lymph node (Figure 3C-D). A fraction of NK cells appeared completely sessile and these cells appeared in most cases to interact with collagen as detected by second harmonic generation (supplemental Video 1). The location of NK cells in the subcapsular area prompted us to visualize CD169+ subcapsular macrophages. To this end, we performed in vivo labeling with a fluorescent anti-CD169 Fab fragment (to avoid FcR engagement on NK cells). Interestingly, we observed that a subset of NK cells displaying reduced motility was closely interacting with CD169+ macrophages (Figure 3E, supplemental Video 2). We found that CD169+ macrophages were no longer detected in the draining lymph node at 48 hours, whereas the bulk of CD11b+ cells were not affected and this effect was independent of IFNAR signaling (supplemental Figure 3). To test whether NK cells were killing SCS macrophages, we examined IL15−/− mice that lack NK cells. We found that SCS macrophages also disappeared in IL15−/− mice on MVA administration (supplemental Figure 3) suggesting that SCS macrophages are not killed by NK cells but instead die due to the cytopathic effects of MVA.14
MVA induces NK cell accumulation in the subcapsular area of the lymph node. NCR1GFP/+ mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. After 16 hours, intact popliteal lymph nodes were subjected to two-photon imaging. (A-B) Representative images from PBS (A) or MVA (B) treated mice. NK cells are shown in green and collagen (2nd harmonic generation) in red. The outline of the lymph node is shown by a dashed line. (C-D) DISC profiles showing the localization of NK cells in PBS (C) or MVA-treated (D) mice and their speed after gating on the indicated area. (E) NCR1GFP/+ mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. At 16 hours, FITC-labeled Fab fragments from an anti-CD169 mAb were injected in the footpad for in vivo labeling of CD169+ SCS macrophages and 1 hour later popliteal lymph nodes were subjected to two-photon imaging. Representative images from PBS and MVA-treated mice (left and middle). NK cells are shown in red, CD169+ cells in green and collagen in white. Tracks of NK cells in MVA treated mice are shown in red (right).
MVA induces NK cell accumulation in the subcapsular area of the lymph node. NCR1GFP/+ mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. After 16 hours, intact popliteal lymph nodes were subjected to two-photon imaging. (A-B) Representative images from PBS (A) or MVA (B) treated mice. NK cells are shown in green and collagen (2nd harmonic generation) in red. The outline of the lymph node is shown by a dashed line. (C-D) DISC profiles showing the localization of NK cells in PBS (C) or MVA-treated (D) mice and their speed after gating on the indicated area. (E) NCR1GFP/+ mice were injected in the footpad with 5 × 106 pfu of MVA or with PBS. At 16 hours, FITC-labeled Fab fragments from an anti-CD169 mAb were injected in the footpad for in vivo labeling of CD169+ SCS macrophages and 1 hour later popliteal lymph nodes were subjected to two-photon imaging. Representative images from PBS and MVA-treated mice (left and middle). NK cells are shown in red, CD169+ cells in green and collagen in white. Tracks of NK cells in MVA treated mice are shown in red (right).
Altogether, these experiments indicate that MVA induces NK cells to accumulate in the subcapsular area of lymph nodes in close proximity to CD169+ cells, presumably SCS macrophages. SCS macrophages are probably among the first cells to encounter lymph-node MVA particles, as reflected by their preferential death after MVA administration.
Subcapsular macrophages are required for activation of NK cell on local administration of MVA but not of poly I:C
The close proximity of NK cells and SCS macrophages suggested a potential role for SCS macrophages in promoting NK cell activation. To test this possibility, we injected clodronate (CL) liposomes in the footpad of naive mice to deplete SCS macrophages in the draining lymph node. As observed previously, we found that CL-treatment resulted in the efficient depletion of CD11b+ CD169+ cells with no apparent effect on CD11b+ CD169− cells in the draining lymph node (Figure 4A-B). As shown in Figure 4C through F, CL-treatment abolished NK cell accumulation as well as the up-regulation of CD69 and intracellular granzyme B in response to MVA. However, NK cell responses were preserved when mice were treated with control (PBS) liposomes. To exclude the possibility that CL-treatment resulted in a nonspecific inhibition of NK cell activation, we injected poly I:C in the footpad of CL-treated mice. We found that NK cells in CL-treated animals responded to poly I:C efficiently, showing normal up-regulation of CD69 and granzyme B (Figure 4E-F). This result indicates that NK cells from CL-treated mice remain responsive to activating signals, excluding a nonspecific effect of CL on NK cell functions.
Subcapsular macrophages are required for NK cell activation by MVA but not by poly I:C. WT mice were treated with PBS or with clodronate (CL-Lipo) or control liposomes (PBS-Lipo) in the footpad to deplete SCS macrophages in the draining popliteal lymph node. (A-B) Depletion of CD169+ cells was assessed by flow cytometry 6 days after liposome treatment. Numbers correspond to percentage of cells falling in the indicated gate. (C-D) PBS-Lipo or CL-Lipo treated mice were injected with MVA (or PBS) in the footpad. (C) NK cell accumulation mediated by MVA was assessed after 24 hours. (D) Intracellular granzyme B content was measured in NK cells (NK1.1+ CD3−). Note that NK cell activation mediated by MVA was strongly reduced in CL-Lipo treated mice. (E-F) CL-Lipo–mediated depletion prevents NK cell activation by MVA but not by poly I:C. The percentage of CD69+ (E) and of Granzyme Bhigh (F) NK cells are shown for the indicated treatments and stimuli. Data are representatative of 3 independent experiments (*P < .05; **P < .01).
Subcapsular macrophages are required for NK cell activation by MVA but not by poly I:C. WT mice were treated with PBS or with clodronate (CL-Lipo) or control liposomes (PBS-Lipo) in the footpad to deplete SCS macrophages in the draining popliteal lymph node. (A-B) Depletion of CD169+ cells was assessed by flow cytometry 6 days after liposome treatment. Numbers correspond to percentage of cells falling in the indicated gate. (C-D) PBS-Lipo or CL-Lipo treated mice were injected with MVA (or PBS) in the footpad. (C) NK cell accumulation mediated by MVA was assessed after 24 hours. (D) Intracellular granzyme B content was measured in NK cells (NK1.1+ CD3−). Note that NK cell activation mediated by MVA was strongly reduced in CL-Lipo treated mice. (E-F) CL-Lipo–mediated depletion prevents NK cell activation by MVA but not by poly I:C. The percentage of CD69+ (E) and of Granzyme Bhigh (F) NK cells are shown for the indicated treatments and stimuli. Data are representatative of 3 independent experiments (*P < .05; **P < .01).
Next, we examined the contribution of SCS macrophages to type I IFN production. We found that MVA resulted in an increased production of type I IFN in the lymph nodes but that CL-treatment abolished this production (supplemental Figure 4). Thus, SCS macrophages are required for type I IFN production in the lymph node. To determine whether type I IFNs were acting directly on NK cells or indirectly on accessory cells, we transferred WT or IFNAR−/− NK cells in either WT or IFNAR−/− host. Activation of WT NK cells was largely impaired in IFNAR−/− recipients suggesting that IFNAR signaling on accessory cells is important for NK cells activation. This observation does not exclude that type I IFN also act on NK cell directly as this defect might be accounted for by the lower amount of type I IFN produced in IFNAR−/− mice.5 In contrast, activation of IFNAR−/− NK cells was preserved in WT recipient indicating that IFNAR signaling on NK cells is not absolutely required for activation by MVA (supplemental Figure 5). These results highlight the importance of type I IFN signaling on accessory cells to promote NK cells activation.
Altogether, our results suggest that CD169+ subcapsular macrophages are critical for NK cell activation by lymph-borne viral particles but dispensable for activation by the double-stranded RNA analog, poly I:C.
Subcapsular macrophages are required for NK cell redistribution and deceleration in SCS areas
Finally, we assessed whether SCS macrophages are required for the observed changes in NK cell distribution and motility in the lymph node draining the site of MVA injection. To this end, we performed two-photon imaging of lymph nodes from CL-treated mice, 16 hours after MVA administration. As shown in Figure 5 and supplemental Video 3, CL-treatment virtually abolished NK cell accumulation in SCS areas (Figure 5A-B). In addition, the marked deceleration of NK cells in SCS areas was also largely attenuated (Figure 5C-D). These results strongly suggest that, in response to MVA, SCS macrophages promote the recruitment and deceleration of NK cells in SCS areas. We propose that SCS macrophages are essential for the NK cell response to lymph-borne viral particles.
Subcapsular macrophages are required for NK cell recruitment in the SCS area and deceleration. NCR1GFP/+ mice were treated with clodronate (CL-Lipo) or PBS and injected with MVA in the footpad. After 24 hours, intact popliteal lymph nodes were subjected to two-photon imaging. (A-B) Representative images showing that NK cell accumulation in the SCS area of the lymph node in response to MVA is lost in CL-Lipo treated mice. (C-D) DISC analyses showing the location and speed of NK cells in control and Lipo-CL treated mice injected with MVA.
Subcapsular macrophages are required for NK cell recruitment in the SCS area and deceleration. NCR1GFP/+ mice were treated with clodronate (CL-Lipo) or PBS and injected with MVA in the footpad. After 24 hours, intact popliteal lymph nodes were subjected to two-photon imaging. (A-B) Representative images showing that NK cell accumulation in the SCS area of the lymph node in response to MVA is lost in CL-Lipo treated mice. (C-D) DISC analyses showing the location and speed of NK cells in control and Lipo-CL treated mice injected with MVA.
Discussion
To gain insight into how the NK cells sense lymph borne viral particles, we visualized and analyzed NK cell activation after subcutaneous administration of a recombinant MVA-based viral vector. Specifically, we showed that NK cells accumulated in the draining lymph node, redistributed in the SCS areas, and closely associated with SCS macrophages and collagen fibers. SCS macrophages were found to be critical for NK cell accumulation and activation in the draining lymph node. We propose that SCS macrophages contribute to antiviral immunosurveillance by sensing lymph-borne viral particles and by recruiting and activating NK cells.
Although NK cells are poised for a rapid response, their effector functions are largely enhanced by cytokines and contacts with accessory cells.1,22,23 Several studies have highlighted a critical role for dendritic cells in mediating NK cell activation.2,24-27 For example, depletion of CD11c+ cells abolished the NK cell response to Listeria or to a variety of TLR agonists.2 IL-15 transpresentation by DCs was found to be involved in DC-mediated NK cell activation in vivo.2,28,29 In addition to DCs, stromal cells can mediate NK cell activation in response to poly I:C.4 However, which accessory cells might promote NK cell activation during infections by lymph-borne viruses remains to be determined.
This work provides in vivo evidence that SCS macrophages play a critical role for NK cell responses to lymph borne viral particles. Depletion of SCS macrophages with clodronate liposomes abolished the enrichment for NK cells in the draining lymph node seen on MVA injection. This enrichment was probably not because of local proliferation as we did not observe dividing NK cells in the course of our imaging experiments. Rather, NK cell accumulation was critically dependent on CXCR3, a result reminiscent of findings made in the context of injection of mature DCs.30 Thus, although viral infections are known to influence NK cell trafficking in the spleen,31-33 we show here that lymph-borne viral particles profoundly alter NK cell numbers and distribution in lymph nodes and that these effects are dependent on SCS macrophages. Moreover, depletion of SCS macrophages abolished NK cell activation in response to MVA but not to poly I:C. Therefore, it appears that distinct cell types contribute to NK cell activation depending on the nature of the stimulus and/or on its route of delivery. Future studies should help determine whether SCS macrophages contribute to NK cell activation seen in response to other pathogens such as Leishmania major.34
MVA is a single-cycle, nonreplicating virus that is currently being used as a candidate vaccine in various clinical trials.12,13 Our results suggest that SCS macrophages could play an important role in the response to such vaccine candidates. In our study, we used a recombinant MVA developed by the ANRS, to elicit T-cell responses against HIV epitopes,14 and which will soon enter into phase 1 clinical trial. Our present work demonstrates that SCS macrophages have a profound impact on the lymph node microenvironment and the innate immune response on MVA administration. Future work will help assessing whether SCS macrophages contribute to the adaptive immune response and the generation of T-cell responses. Moreover, our approach illustrates how in vivo imaging allows dissecting the early effects and mode of action of vaccine candidates with a high spatiotemporal resolution.
Importantly, our results extend the list of recently identified functions for SCS macrophages. Indeed, these cells have been shown to present viral particles to B cells11 and to prevent vesicular stomatitis virus (VSV) from gaining access to peripheral nerves.35 SCS macrophages have also been implicated in antigen-presentation to T cells in response to vaccinia virus,36 Toxoplama gondii37 or tumors,38 and in NKT cell activation by lipid antigen.39 Thus, by sensing lymph content in lymph nodes, SCS macrophages appear to play a pivotal role in the initiation of both innate and adaptive immune responses.
Imaging the dynamics of MVA-induced NK cell activation revealed striking differences with the behavior of NK cells after systemic delivery of poly I:C that we previously studied.10 In response to poly I:C, we observed that NK cells redistributed toward the center of the lymph node, while maintaining high motility. In sharp contrast, subcutaneous delivery of MVA promoted the accumulation of NK cells displaying low motility in the subcapsular area. One possible explanation for these distinct localizations may originate from the difference in cells targeted by these stimuli (SCS macrophages versus DCs) that might translate into distinct distribution of inflammatory chemokines in the lymph node. In this respect, it has been shown that macrophages use TLR2-TLR6, MDA-5, and the NALP3 inflammasome to sense MVA and produce type I IFN and several chemokines including CXCL10 and CCL5.15 Consistent with data showing that MVA can infect macrophages and induce apoptosis of infected cells,14 we noted a progressive disappearance of SCS macrophages in the draining lymph node of MVA injected mice.
What is the origin of NK cell deceleration in the subcapsular area? We found that a subset of NK cells were completely immotile and bound to collagen fibers as detected by second harmonic generation. This behavior might be reminiscent of work by Garrod et al showing that activated NK cells can bind to collagen fibers in the lymph node through engagement of CD49b.40 Another subset of slowly migrating NK cells was found to be in close proximity to SCS macrophages. Whether this association is the result of receptor-ligand interactions or of chemokine-mediated NK cell attraction and retention remains to be characterized. At any rate, such proximity probably favors the delivery of cytokines, such as type I IFN, at high local concentration to NK cells. Indeed, SCS macrophages have been shown to be responsible for a large fraction of type I IFN production in response to lymph-borne viruses,35 and we and others have previously shown that direct action of type I IFN on NK cells may favor optimal NK cell activation in vivo.5 In addition, other cytokines and/or receptor-ligand interactions may be provided during SCS macrophage-NK cell contacts and contribute to NK cell activation.
In summary, we report that lymph-borne viral particles induce a strong NK cell response that is critically dependent on type I IFN and on the presence of SCS macrophages. We propose that SCS macrophages contribute to antiviral immunity and to the response to viral-based vaccines by surveying lymph for circulating viruses and rapidly promoting NK cell accumulation, redistribution, and activation on virus detection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Bousso laboratory for helpful comments on the manuscript and James Di Santo for providing IL-15−/− mice. Cl2MDP was a gift of Roche Diagnostics GmbH.
This work was supported by ANRS, Institut Pasteur, Inserm, Fondation pour la Recherche Médicale, and a European Research Council Starting Grant (LymphocyteContacts).
Authorship
Contribution: Z.G. designed and performed research and analyzed data; F.L. performed research; N.R. provided critical reagents; Y.L. designed research; M.L.A. and O.S. provided critical reagents and designed research; and P.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Phillipe Bousso, Institut Pasteur, Dynamics of Immune Responses Unit, 25 rue du Dr Roux, Paris, France 75015; e-mail: philippe.bousso@pasteur.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal