Abstract
Recruitment and retention of leukocytes at a site of blood vessel growth are crucial for proper angiogenesis and subsequent tissue perfusion. Although critical for many aspects of regenerative medicine, the mechanisms of leukocyte recruitment to and actions at sites of angiogenesis are not fully understood. In this study, we investigated the signals attracting leukocytes to avascular transplanted pancreatic islets and leukocyte actions at the engraftment site. Expression of the angiogenic stimulus VEGF-A by mouse pancreatic islets was elevated shortly after syngeneic transplantation to muscle. High levels of leukocytes, predominantly CD11b+/Gr-1+/CXCR4hi neutrophils, were observed at the site of engraftment, whereas VEGF-A–deficient islets recruited only half of the amount of leukocytes when transplanted. Acute VEGF-A exposure of muscle increased leukocyte extravasation but not the levels of SDF-1α. VEGF-A–recruited neutrophils expressed 10 times higher amounts of MMP-9 than neutrophils recruited to an inflammatory stimulus. Revascularization of islets transplanted to MMP-9–deficient mice was impaired because blood vessels initially failed to penetrate grafts, and after 2 weeks vascularity was still disturbed. This study demonstrates that VEGF-A recruits a proangiogenic circulating subset of CD11b+/Gr-1+ neutrophils that are CXCR4hi and deliver large amounts of the effector protein MMP-9, required for islet revascularization and functional integration after transplantation.
Introduction
Over the last decade, an important role for leukocytes in vascular development and maintenance has emerged. Early clinical histopathologic evidence of a correlation between leukocyte infiltration in tumors and poor survival is now explained by certain leukocytes contributing to tumor vascularization required for tumor growth. Indeed, recent data demonstrate considerable effects of immune cells in angiogenesis,1 and proangiogenic effects of monocytes,2,3 macrophages,4 and neutrophils5-7 have been demonstrated using different experimental settings. Leukocytes thus act in concert with other cell types like endothelial progenitor cells,8 pericytes9 and mature endothelial cells to create microenvironmental signals that make up a complex proangiogenic interactive network.
In a model of β-cell replacement for the cure of type 1 diabetes, we recently showed that Gr-1+ leukocytes, mostly neutrophils, were indispensible for the development of a new vascular network in transplanted pancreatic islets in muscle.10 These findings have now been transferred into clinical development. During infection, neutrophil activation results in changed surface antigen expression and neutrophil function.11-13 Whether neutrophils with distinct proangiogenic phenotypic characteristics are specifically recruited to hypoxic situations is not yet known. Indeed, the role of neutrophils in angiogenesis is not yet well established, but they have recently been shown to be important for endometrial14 and tumor6 angiogenesis and to be responsible for initiation of the angiogenic switch in the RIP1-Tag2 model of pancreatic carcinoma.5 In the latter study, it was also found that neutrophils present in the angiogenic lesions expressed high levels of the enzyme matrix metalloproteinase-9 (MMP-9).
An element that further suggests a critical role for neutrophils and MMP-9 in blood vessel growth is that they are the sole cell type in the body that produces and secretes MMP-9 without its endogenous inhibitor tissue inhibitor of metalloproteinases-1.15,16 Also called gelatinase B, MMP-9 is the most complex member of the enzyme family of matrix metalloproteinases and plays important roles in several physiologic and pathophysiologic events.17 For instance, MMP-9 degrades components of the extracellular matrix facilitating tissue remodeling and releasing, thereby activating bound growth factors, such as vascular endothelial growth factor-A (VEGF-A).17-19 MMP-9 is also involved in the regulation of leukocytosis20 (eg, by potentiating at least 10-fold the proangiogenic and neutrophil attracting IL-821 ) and by the release of hematopoietic progenitor cells from the bone marrow.22,23
How circulating leukocytes are recruited to infection or inflammation is well charted, but the cue that directs proangiogenic leukocytes to a site of angiogenesis is not fully understood. However, emerging evidence exists for the involvement of the hypoxia-inducible factor 1-α and its downstream products like stromal cell-derived factor-1α (SDF-1α/CXCL12) and VEGF-A in recruitment and retention of leukocytes in angiogenic environments.24-28 The β-cells of the islet of Langerhans are reported to endogenously produce high levels of the growth factor VEGF-A to uphold the dense angioarchitecture and the fenestrated phenotype of islet capillaries.29-31
In this study, we used nonvascularized transplanted pancreatic islets as a model of angiogenesis, explored the signals attracting leukocytes to a site of vascular development, and studied the identities of these proangiogenic leukocytes. A role for VEGF-A in leukocyte recruitment to sites of hypoxia was demonstrated because a CD11b+/Gr-1+/CXCR4hi leukocyte subset was efficiently recruited from the circulation. This neutrophil subset deliver considerable amounts of the angiogenic effector protein, MMP-9, which was shown to be important in the revascularization of transplanted pancreatic islets.
Methods
Animals
Male C57Bl/6 mice (25-30 g; Taconic), MMP-9-deficient (MMP-9−/−) mice, backcrossed 12 generations to C57Bl/6 (25-30 g; Rega Institute)32 and pdx1-cre; VEGFfl/fl (VEGF-A−/−) mice (25-30 g; Vanderbilt University, Nashville, TN)31,33 had free access to tap water and pelleted food throughout the study. All experiments were approved by the Uppsala Laboratory Animal Ethical Committee.
Intravital microscopy of leukocyte recruitment
Mice were anesthetized by spontaneous inhalation of isoflurane (Abbott). The left cremaster muscle was exposed and mounted for intravital microscopic observation. The muscle was continuously superperfused with a bicarbonate-buffered saline solution (37°C, pH 7.4). A catheter in the femoral artery allowed retrograde infusion close intra-arterially to the muscle.
An intravital microscope (Leica Microsystems DM5000B, with a Hamamatsu Orca-R2 CCD camera and a HCX ApoL 20×/0.50W objective, Volocity software) was used to visualize the microcirculation of postcapillary venules and surrounding muscle tissue. Recordings were made for analysis of adherent (stationary for > 30 seconds within 100 μm venule in 3 minutes) and emigrated leukocytes (cells in the extravascular space per field of view, 0.05 mm2).
Recombinant murine MIP-2 (CXCL2, R&D Systems) or VEGF-A (PeproTech) was added to the bicarbonate buffer to induce leukocyte recruitment in the cremaster preparation in a concentration (0.5nM and 2nM, respectively) previously shown to recruit quantifiable numbers of leukocytes to enable visualization and tracking of each leukocyte.34
Peritoneal lavage
Mice were injected intraperitoneally with MIP-2/CXCL2 (30 ng) or VEGF-A (80 ng) in 300 μL saline. After 21 hours, the mice were killed, and 5 mL of HBSS (Sigma-Aldrich) was injected intraperitoneally. The abdomen was massaged for 5 minutes before the cell suspension in HBSS was collected. The lavage fluid was centrifuged at 1500g for 5 minutes, and the pellet was resuspended in PBS.
Single-cell suspension of islet grafts
Islet grafts in muscle and control muscle tissue were excised 1, 2, and 14 days after transplantation. Single-cell suspensions were prepared as previously described.35 Briefly, muscle tissues were minced and placed in collagenase II, 500 U/mL (Sigma Aldrich) in HBSS, 250mM CaCl2 for 30 minutes in 37°C. After a PBS wash, the remaining tissue was placed in collagenase D, 1.5 U/mL (Roche Diagnostics), dispase II, 2.4 U/mL (Sigma-Aldrich) in HBSS, and 250mM CaCl2 for 60 minutes in 37°C. The homogenate was then transferred through a 40-μm cell strainer.
Flow cytometry
Recruited leukocytes were analyzed using a FACSCalibur flow cytometer with CellQuest Pro software (BD Biosciences). Fluorescence compensation was performed using single-labeled samples. Leukocytes were used both unfixed, and fixed and permeabilized (Cytofix/Cytoperm, BD Biosciences), and stained for surface markers with monoclonal antibodies (mAbs) against CD11b (M1/70), Gr-1 (RB6-8C5), CXCR4 (2B11, all eBioscience), and Ly6G (1A8, BD Biosciences Pharmingen) and for intracellular presence of MMP-9 with a polyclonal antibody (R&D Systems). Initially, a gate was placed in forward scatter/side scatter to exclude debris. The population expressing both CD11b and Gr-1 was gated and analyzed for CXCR4 or MMP-9 positivity. Isotype antibodies or normal goat serum were used as controls.
Pancreatic islet isolation and transplantation
Mouse islets were isolated as described earlier.36 Briefly, the mice were anesthetized with 100 mg/kg pentobarbital sodium (Apoteket). Ice-cold collagenase A solution from Clostridium histolyticum, 2.5 mg/mL (Roche Diagnostics) in HBSS was then injected into the pancreas via the common bile duct. Thereafter, the pancreas was removed and placed in a 37°C water bath for 18 minutes. Islets were separated from exocrine tissue by density gradient centrifugation (Histopaque-1077 and RPMI 1640, Sigma-Aldrich). Purified islets were then hand-picked and maintained free-floating in islet culture medium: RPMI 1640 with added D-glucose (11.1mM), l-glutamine (2mM; Sigma-Aldrich), benzyl penicillin (100 U/mL, Roche Diagnostics), streptomycin (0.1 mg/mL), and 10% (volume/volume) FCS (Sigma-Aldrich).
Islets were fluorescently labeled with the intracellular probe Celltracker Blue CMAC (Invitrogen) immediately before transplantation through a butterfly needle to either the cremaster muscle or the abdominal external oblique muscle of syngeneic mice anesthetized with avertin: 2.5% (volume/volume) 2,2,2-tribromoethanol (Sigma-Aldrich) in 2-methyl-2-butanol (Kemila). Fluorescent microspheres (125-150 μm, Cospheric) were transplanted as the islets.
VEGF-A and SDF-1α protein measurements
The VEGF-A (islet grafts and muscle) and SDF-1α (superperfused cremaster muscles) proteins were measured. Tissues were excised, snap-frozen in liquid nitrogen, and homogenized in RIPA-buffer, and thereafter the manufacturer's protocol was followed. An electrochemoluminescence method was used for VEGF-A (mouse/rat VEGF tissue culture kit, Meso Scale Discovery) and analyzed on a SECTOR Imager 2400 (Meso Scale Discovery), and an ELISA made for SDF-1α (Quantikine, R&D Systems) was analyzed on a Safire2 plate reader (Tecan).
Laser scanning confocal microscopy
Transplanted and anesthetized mice received anti–CD31-mAb (360, eBioscience) conjugated to AlexaFluor-555 (Invitrogen) and anti–Gr-1-mAb (RB6-8C5, eBioscience) conjugated to FITC or eFluor 660 intra-arterially. Confocal imaging of grafts, vasculature, and leukocytes was performed using laser scanning confocal microscopes: Zeiss 5 Live on an AxioImager Z1 base with a Plan-Apo 40×/1.0 W objective (0.5-2.0× optical zoom) and Zeiss Zen 2011 software, or Nikon C1 on a TE2000-U base with a Plan-Apo VC 20×/0.75 W objective and Nikon EZ-C1 Version 3.90 software.
Immunohistochemistry
Muscles containing islet grafts were snap-frozen in liquid nitrogen, cryosectioned, stained for blood vessels with AlexaFluor-555–CD31-mAb and for leukocytes with FITC–Gr-1-mAb. Nuclei were stained with Hoechst 33345 (Invitrogen), and images quantified using ImageJ.
Zymography
Leukocytes recruited to the peritoneum by either MIP-2/CXCL2 or VEGF-A were placed in 24-well plates in 2 different concentrations (2 × 104 and 4 × 104 leukocytes/mL), and phorbol-12-myristate-13-acetate (PMA, 1-100 ng/mL) was added as secretagogue before incubation at 37°C for 1 hour. Media were harvested for gelatin zymography analysis to determine the levels of gelatinases (gelatinase A/MMP-2 and gelatinase B/MMP-9). Both gelatinases in the cell supernatants were prepurified by binding to gelatin-Sepharose as described37 and loaded onto 0.1% SDS/7.5% polyacrylamide gels containing 0.1% gelatin. Electrophoresis was performed in Tris-glycine buffer with 0.1% SDS, where after the gels were washed twice for 30 minutes with 2.5% Triton X-100 to remove SDS, and incubated overnight at 37°C in incubation buffer (50mM Tris-HCl, pH 7.5, 10mM CaCl2, 0.02% NaN3, 1% Triton X-100) for gelatin degradation. Gelatinase levels were revealed by staining with 0.25% Coomassie Brilliant Blue R-250, 45% methanol and 10% acetic acid, and destaining with 30% methanol and 10% acetic acid.
In situ zymography
Local gelatinolytic activity was developed on 7-μm-thick cryosections of grafts in muscle (protocol modified from Oh et al38 ). The sections were incubated for 4 hours at 37°C with 20 μg/mL–quenched FITC-labeled gelatin (DQ gelatin, Invitrogen) in saline buffer. After incubation, images were acquired using a confocal microscope. Addition of 15mM EDTA (as a chelating agent that inhibits metal ion dependent proteases) to the assay solution served as negative control.
Statistics
Values are expressed as mean ± SEM. Paired or unpaired 2-tailed Student t tests or Mann-Whitney nonparametric test were used for comparison between groups.
Results
Pancreatic islets transplanted into muscle produce high levels of VEGF-A
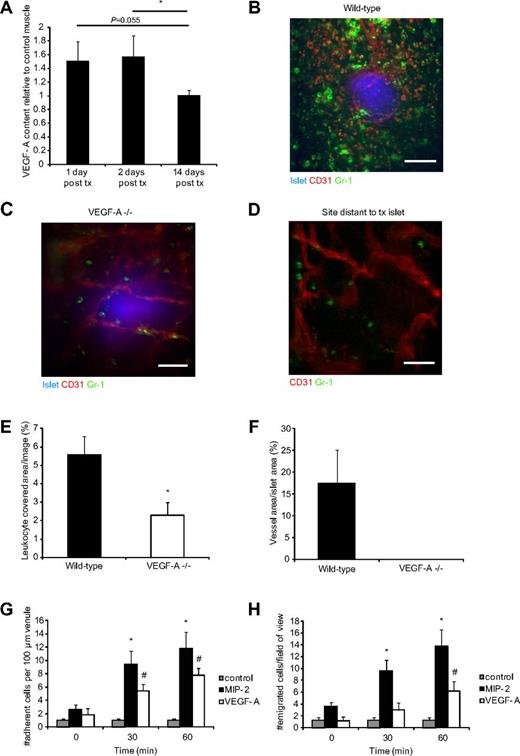
In our observations of syngeneically transplanted pancreatic islets into striated muscle, we found an abundance of Gr-1+ leukocytes in the perigraft area shortly after transplantation,10 which is not the result of acute immunologic rejection because the islets originate from donors genetically identical to the recipients. Further, extravasated leukocytes are not found after sham injections of islet culture medium (transplantation vehicle) or after transplantation of polyethylene spheres (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), demonstrating that it is the transplanted graft and not the insult per se that is responsible for recruitment of leukocytes. β-cells in pancreatic islets are able to produce high levels of the hypoxia-induced growth factor VEGF-A,29,31 which was increased further in isolated islets cultured for 1 day (supplemental Figure 2). In vivo, muscle with engrafting islets expressed 60% higher levels of VEGF-A 1 and 2 days after transplantation compared with control muscle but returned to background levels at 14 days after transplantation (Figure 1A) when revascularization was completed, indicating a hypoxia response during the avascular period during engraftment.
VEGF-A secreted from transplanted islets recruits Gr-1+ leukocytes. (A) VEGF-A protein measurements in islet grafts in abdominal muscle 1 (n = 5), 2 (n = 6), and 14 (n = 5) days after transplantation (tx). Values are expressed as relative to the VEGF-A content in abdominal muscle on the contralateral side of the linea alba. *P < .05. (B) Confocal z-projection image of a transplanted wild-type pancreatic islet (blue, Celltracker) in the cremaster muscle of a wild-type mouse 4 days after transplantation. Blood vessels (red, CD31-mAb) surround and penetrate the islet, and Gr-1+ leukocytes (green, Gr-1-mAb) are gathered around the graft. Bar represents 50 μm. (C) Confocal z-projection image of a VEGF-A−/− islet 4 days after transplantation to a wild-type mouse cremaster muscle with few surrounding leukocytes and no ingrowing blood vessels. The blood vessels that are visible lie beneath the graft. Bar represents 50 μm. (D) Confocal z-projection image showing a site in a wild-type cremaster muscle distant from a transplanted islet illustrating the background level of leukocyte extravasation in these tissues. Bar represents 50 μm. (E) Quantification of the Gr-1+ area per confocal image of islet grafts 3-5 days after transplantation. VEGF-A−/− islets (n = 8 mice, 14 islets) had less than half of the amount of recruited leukocytes compared with wild-type islets (n = 8 mice, 15 islets). *P < .05. (F) Vessel densities in the transplanted islets from wild-type and VEGF-A−/− mice. (G) Numbers of leukocytes that adhere to a 100-μm part of postcapillary venules in the cremaster muscle after stimulation with MIP-2 (n = 5), VEGF-A (n = 5), or bicarbonate buffer (control, n = 3). *P < .05, compared with time 0 minutes for MIP-2. #P < .05, compared with time 0 minutes for VEGF-A. (H) Numbers of leukocytes that emigrate from postcapillary venules into the cremaster muscle after stimulation with MIP-2 (n = 5), VEGF-A (n = 5), or bicarbonate buffer (control, n = 3). *P < .05, compared with time 0 minutes for MIP-2. #P < .05, compared with time 0 minutes for VEGF-A.
VEGF-A secreted from transplanted islets recruits Gr-1+ leukocytes. (A) VEGF-A protein measurements in islet grafts in abdominal muscle 1 (n = 5), 2 (n = 6), and 14 (n = 5) days after transplantation (tx). Values are expressed as relative to the VEGF-A content in abdominal muscle on the contralateral side of the linea alba. *P < .05. (B) Confocal z-projection image of a transplanted wild-type pancreatic islet (blue, Celltracker) in the cremaster muscle of a wild-type mouse 4 days after transplantation. Blood vessels (red, CD31-mAb) surround and penetrate the islet, and Gr-1+ leukocytes (green, Gr-1-mAb) are gathered around the graft. Bar represents 50 μm. (C) Confocal z-projection image of a VEGF-A−/− islet 4 days after transplantation to a wild-type mouse cremaster muscle with few surrounding leukocytes and no ingrowing blood vessels. The blood vessels that are visible lie beneath the graft. Bar represents 50 μm. (D) Confocal z-projection image showing a site in a wild-type cremaster muscle distant from a transplanted islet illustrating the background level of leukocyte extravasation in these tissues. Bar represents 50 μm. (E) Quantification of the Gr-1+ area per confocal image of islet grafts 3-5 days after transplantation. VEGF-A−/− islets (n = 8 mice, 14 islets) had less than half of the amount of recruited leukocytes compared with wild-type islets (n = 8 mice, 15 islets). *P < .05. (F) Vessel densities in the transplanted islets from wild-type and VEGF-A−/− mice. (G) Numbers of leukocytes that adhere to a 100-μm part of postcapillary venules in the cremaster muscle after stimulation with MIP-2 (n = 5), VEGF-A (n = 5), or bicarbonate buffer (control, n = 3). *P < .05, compared with time 0 minutes for MIP-2. #P < .05, compared with time 0 minutes for VEGF-A. (H) Numbers of leukocytes that emigrate from postcapillary venules into the cremaster muscle after stimulation with MIP-2 (n = 5), VEGF-A (n = 5), or bicarbonate buffer (control, n = 3). *P < .05, compared with time 0 minutes for MIP-2. #P < .05, compared with time 0 minutes for VEGF-A.
Transplanted islets deficient in VEGF-A recruit fewer leukocytes
To investigate the effects of islet-produced VEGF-A on the recruitment of leukocytes after transplantation, islets isolated from mice deficient in β-cell VEGF-A (pdx1-cre; VEGFfl/fl, here called VEGF-A−/−) were transplanted to cremaster muscles of wild-type mice of the same background. Grafts were examined 3, 4, or 5 days after transplantation by in vivo confocal microscopy, and quantifications revealed a 59% decrease in Gr-1+ leukocyte recruitment to VEGF-A–deficient islets (pdx1-cre; VEGFfl/fl, Figure 1C) compared with control (VEGFfl/fl, Figure 1B) islets (2.9% ± 0.7% vs 5.6% ± 0.9% leukocyte coverage/islet tissue, respectively; Figure 1E). The number of extravasated leukocytes in the vicinity of the transplanted VEGF-A−/− islets was about the same as the background level of leukocytes at sites distant to transplanted islets in most cremaster muscles (Figure 1D), further demonstrating that the leukocyte accumulation at VEGF-A–producing islet grafts was not caused by surgical trauma. No blood vessels could be found in VEGF-A−/− islets, whereas wild-type islets showed intense angiogenesis during this time period (Figure 1F).
Recombinant VEGF-A is capable of inducing rapid leukocyte recruitment
The findings using transplanted islets describe a process of leukocyte recruitment over several days. To study the short-time kinetics of VEGF-A–induced leukocyte recruitment, the mouse cremaster muscle was superperfused in vivo with recombinant VEGF-A in physiologically relevant concentrations. The recruitment of leukocytes was observed through an intravital microscope and compared with that achieved by superperfusion with the inflammatory chemokine MIP-2 (CXCL2). MIP-2, previously shown to induce recruitment of predominantly neutrophils in the cremaster model of inflammation,39 caused increased adhesion of leukocytes to postcapillary venular walls (Figure 1G) and emigration into muscle tissue (Figure 1H), as expected. More intriguing was the finding that VEGF-A induced rapid onset of leukocyte recruitment and showed high numbers of both adherent (Figure 1G) and emigrated (Figure 1H) leukocytes already after 30 minutes of superperfusion (observed only in postcapillary venules and not in arterioles). Although de novo synthesis of secondary messengers during the time period studied is improbable, the amount of SDF-1α protein was analyzed in the cremaster muscle after bicarbonate buffer superperfusion (0.39 ± 0.1 pg SDF-1α/mL homogenate) and VEGF-A superperfusion (0.39 ± 0.05 pg SDF-1α/mL homogenate). These results demonstrated that the VEGF-A–induced leukocyte recruitment was not the result of up-regulation of SDF-1α but may be a direct effect of VEGF-A on leukocytes or endothelium.
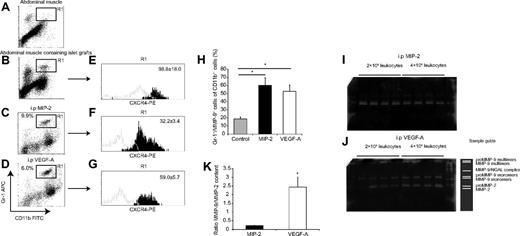
Islet grafts and recombinant VEGF-A recruit CD11b+/Gr-1+/CXCR4hi/MMP-9+ leukocytes
Next, the identity of leukocytes recruited to transplanted islets and to recombinant VEGF-A was investigated and compared with a population of leukocytes recruited during infection-like conditions by MIP-2. Grafts of 300 pancreatic islets transplanted 4 days earlier and surrounding abdominal muscle tissue were collected and dispersed into single-cell suspensions using enzymatic degradation. To attain quantifiable amounts of leukocytes in a nontransplantation setting, peritoneal lavages were performed after intraperitoneal injection of either MIP-2 or VEGF-A. Resident and infiltrated leukocytes were retrieved, counted, fixed, and analyzed by flow cytometry (Figure 2). Approximately 40% of the cells present in the peritoneal lavages in response to either MIP-2 or VEGF-A were positive for the myeloid marker CD11b. Of these cells, the Gr-1+ population, which we previously found to be important for islet angiogenesis,10 was gated and analyzed with regard to CXCR4 expression and MMP-9 content. The CXCR2-ligand MIP-2 was, as expected, efficient in recruiting Gr-1+ leukocytes, whereas in the saline control situation, this cell type was scarcely present in the lavages (data not shown). Interestingly, VEGF-A had the capability of recruiting these CD11b+/Gr-1+ leukocytes in amounts comparable with those recruited to MIP-2 (Figure 2C-D). The Gr-1 epitope is present on several myeloid subsets. Therefore, we also tested for the Ly6G epitope, primarily present on granulocytes.40 There was a complete overlap in Ly6G-positivity of the CD11b+/Gr-1+ population (data not shown). Leukocytes recruited to islet grafts in muscle or by intraperitoneal recombinant VEGF-A expressed significantly higher amounts of the SDF-1α receptor CXCR4 compared with leukocytes recruited to MIP-2 (Figure 2E-G). We then investigated whether CXCR4 levels on circulating neutrophils could be altered by exposure of MIP-2 or VEGF-A for 30 minutes, and we found no detectable differences in receptor levels by flow cytometric analysis (supplemental Figure 3A), indicating the preexistence of circulating neutrophil subsets that differ in CXCR4 expression. To assess whether changed settings could influence the CXCR4 levels, leukocytes from MIP-2 or VEGF-A intraperitoneal lavages were incubated with MIP-2, VEGF-A, or cell medium for 30 minutes. This in vitro treatment did not induce any detectable changes in surface expression of CXCR4 after normalization to that of untreated controls (supplemental Figure 3B), suggesting that, during this time, CXCR4 expression is static and the observed differences may be native to these cells.
VEGF-A recruits a subset of CD11b+/Gr-1+/CXCR4hi neutrophils that contain large amounts of MMP-9. (A-B) Flow cytometric plots from single-cell suspensions of islet grafts in abdominal muscle and muscle from the contralateral side of the linea alba (n = 4). (C-D) Flow cytometric plots from cells harvested from peritoneal lavages where leukocytes were recruited to intraperitoneal (i.p.) injections of either MIP-2 or VEGF-A (n = 5 in each group). Both stimuli recruited similar levels of CD11b/Gr-1+ leukocytes (R1 gate). (E-G) The R1 gates from the plots in panels B through D were further analyzed for CXCR4 expression. The histograms represent fluorescence intensity from the CXCR4-mAb (black) and the isotype control-mAb (gray). Numbers in histograms are the mean fluorescence intensities with subtracted isotype intensities. The CXCR4 expression in leukocytes from islet grafts and intraperitoneal VEGF-A is statistically different from the leukocytes recruited to intraperitoneal MIP-2 (P < .05). (H) CD11b/Gr-1 double-positive cells from panels C and D were again gated and analyzed for MMP-9-content and compared with saline control animals (n = 5 in each group). *P < .05, compared with control. (I-J) Total leukocytes from peritoneal lavages were plated in different cell concentrations in the presence of PMA for 1 hour, and the media were then harvested. Images show representative full gels from 1 gelatin zymography experiment. (K) Leukocytes recruited to VEGF-A contained more MMP-9 than leukocytes recruited to MIP-2 in relation to MMP-2 content (n = 3 separate experiments per group).*P < .05.
VEGF-A recruits a subset of CD11b+/Gr-1+/CXCR4hi neutrophils that contain large amounts of MMP-9. (A-B) Flow cytometric plots from single-cell suspensions of islet grafts in abdominal muscle and muscle from the contralateral side of the linea alba (n = 4). (C-D) Flow cytometric plots from cells harvested from peritoneal lavages where leukocytes were recruited to intraperitoneal (i.p.) injections of either MIP-2 or VEGF-A (n = 5 in each group). Both stimuli recruited similar levels of CD11b/Gr-1+ leukocytes (R1 gate). (E-G) The R1 gates from the plots in panels B through D were further analyzed for CXCR4 expression. The histograms represent fluorescence intensity from the CXCR4-mAb (black) and the isotype control-mAb (gray). Numbers in histograms are the mean fluorescence intensities with subtracted isotype intensities. The CXCR4 expression in leukocytes from islet grafts and intraperitoneal VEGF-A is statistically different from the leukocytes recruited to intraperitoneal MIP-2 (P < .05). (H) CD11b/Gr-1 double-positive cells from panels C and D were again gated and analyzed for MMP-9-content and compared with saline control animals (n = 5 in each group). *P < .05, compared with control. (I-J) Total leukocytes from peritoneal lavages were plated in different cell concentrations in the presence of PMA for 1 hour, and the media were then harvested. Images show representative full gels from 1 gelatin zymography experiment. (K) Leukocytes recruited to VEGF-A contained more MMP-9 than leukocytes recruited to MIP-2 in relation to MMP-2 content (n = 3 separate experiments per group).*P < .05.
VEGF-A–recruited leukocytes contain more MMP-9 than leukocytes recruited to an inflammatory stimulus
The CD11b+/Gr-1+ cells recruited to intraperitoneal MIP-2 or VEGF-A also contained MMP-9 to a greater extent than the few resident CD11b+/Gr-1+ cells in the peritoneal cavity from the saline control (Figure 2H). Flow cytometric data revealed presence of MMP-9 within cells, but neither the absolute amount nor the activity of this enzyme was assessed by this method. To address the levels of MMP-9 that were found in the subpopulation of the recruited leukocytes, gelatin zymography analysis was performed on supernatants from intraperitoneal leukocytes recruited to either MIP-2 or VEGF-A. Leukocytes were incubated in the presence of PMA to stimulate neutrophil degranulation. The zymograms revealed that various concentrations of PMA (1-100 ng/mL) all resulted in maximal MMP-9–release from the leukocytes, and data from all concentrations were therefore pooled. Significant differences in MMP-9–release from leukocyte populations recruited by the 2 different stimuli were observed, despite the use of similar amounts of recruited CD11b+/Gr-1+ cells. Equivalent numbers of cells recruited to VEGF-A released > 10 times more MMP-9 than cells recruited to MIP-2, indicating differences in their phenotype and ability to release this gelatinase (Figure 2I-K; supplemental Figure 4). The content of the constitutively expressed MMP-2 was similar in the both groups, suggesting that the cells in the lavages were equivalent before isolation and similarly processed.15,17 Furthermore, this constitutive presence of MMP-2 allowed us to compare MMP-9 levels between cells induced by MIP-2 and VEGF-A and to express these levels as a ratio between MMP-9 and MMP-2 (Figure 2K).
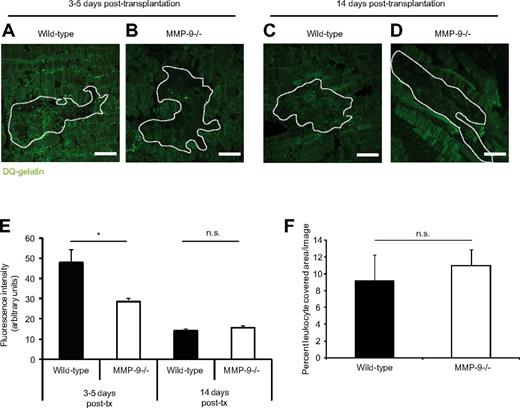
High activity of gelatinases in the area surrounding islet grafts
To investigate whether the in vitro observations on MMP-9 alterations were relevant in vivo when Gr-1+ leukocytes were recruited to transplanted islets during engraftment, the presence and activity of gelatinases were examined 3-5 and 14 days after transplantation in the perigraft area of islets transplanted to muscle with the use of DQ-gelatin conversion. This gelatin form contains quenched FITC that fluoresces when the DQ-gelatin is degraded. The studied time points were based on our previous observations that angiogenesis occurs early after transplantation (eg, 3-5 days) and is completed by day 14 after transplantation.10 Tissue sections of wild-type grafts in wild-type and MMP-9−/− mice 3-5 days after transplantation were incubated with DQ-gelatin and levels of fluorescence were detected by fluorescence microscopy (Figure 3A-B). All sections were positive for enzyme activity, although the mice lacking MMP-9 had significantly lower fluorescence intensity in the area in and nearby the grafts (Figure 3E). The background signal visible in the MMP-9−/− mice is the result of the summed activities of the constitutively expressed gelatinase A (MMP-2) and other enzymes with gelatinase activity. When repeating the assay on tissue sections of 14-day-old grafts, the level of enzyme activity was much lower and the signal intensities from wild-type and MMP-9−/− mice were similar (Figure 3C-E). When staining the slides for Gr-1+ cells, no difference in amounts of recruited leukocytes could be detected in wild-type or MMP-9−/− muscles (Figure 3F). This indicates that leukocyte recruitment to the graft area is functional in both types of animals, but the lack of leukocyte-delivered MMP-9 in the knockout mice results in a lower fluorescence signal compared with the signals in wild-type mice at 3-5 days.
High gelatinase activity in the engraftment area after islet transplantation. (A-B) Confocal images of tissue cryosections of islet grafts 4 days after transplantation to mouse abdominal muscle. (C-D) Islet grafts 14 days after transplantation. The sections were incubated at 37°C with quenched FITC-gelatin. Grafts are outlined by dashed lines. (A) Higher fluorescence intensity in islets engrafted in a wild-type muscle demonstrating high gelatinase activity cleaving the quenched gelatin, emitting absorbed light. (B) Low fluorescence intensity in islets engrafted in an MMP-9−/− mouse. (C-D) The fluorescence intensity was low 14 days after transplantation, and there was no difference between the genotypes. Bars represent 50 μm. (E) Quantification of the fluorescent signal from the cleaved gelatin in the graft area at 3-5 days and 14 days after transplantation. *P < .05. n = 4 in each group. (F) Gr-1 staining of the engrafted islets at 3-5 days after transplantation revealed no significant difference between the groups, indicating that the difference in gelatinase activity is not the result of altered leukocyte recruitment. n.s. indicates not significant.
High gelatinase activity in the engraftment area after islet transplantation. (A-B) Confocal images of tissue cryosections of islet grafts 4 days after transplantation to mouse abdominal muscle. (C-D) Islet grafts 14 days after transplantation. The sections were incubated at 37°C with quenched FITC-gelatin. Grafts are outlined by dashed lines. (A) Higher fluorescence intensity in islets engrafted in a wild-type muscle demonstrating high gelatinase activity cleaving the quenched gelatin, emitting absorbed light. (B) Low fluorescence intensity in islets engrafted in an MMP-9−/− mouse. (C-D) The fluorescence intensity was low 14 days after transplantation, and there was no difference between the genotypes. Bars represent 50 μm. (E) Quantification of the fluorescent signal from the cleaved gelatin in the graft area at 3-5 days and 14 days after transplantation. *P < .05. n = 4 in each group. (F) Gr-1 staining of the engrafted islets at 3-5 days after transplantation revealed no significant difference between the groups, indicating that the difference in gelatinase activity is not the result of altered leukocyte recruitment. n.s. indicates not significant.
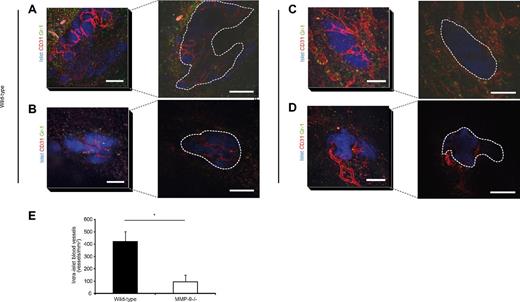
Newly formed blood vessels in MMP-9−/− mice fail to penetrate islet grafts
To investigate the relevance of MMP-9 in revascularization of transplanted islets, mouse islets were isolated from wild-type mice and transplanted to the cremaster muscle (for intravital imaging) and abdominal external oblique muscle (for immunohistochemistry) of wild-type or MMP-9−/− mice. Vasculature and recruited Gr-1+ leukocytes were detected by intra-arterial injection of fluorescently labeled antibodies (anti-CD31 and anti–Gr-1, respectively) and the engraftment area was visualized by confocal microscopy. Islets in wild-type mice were rapidly revascularized 3-5 days after transplantation (Figure 4A-B) as we previously have reported.10 The grafts in MMP-9−/− mice also showed early angiogenesis with host vessels being adjacent to the perimeter of transplanted islets at early time points after transplantation (Figure 4C-D). However, optical sectioning of the tissue revealed that islet grafts in MMP-9−/− recipients had fewer intra-islet capillaries 3-5 days after transplantation (Figure 4C-D right panels), compared with wild-types (Figure 4A-B right panels). This was also confirmed in cryosections, from which we quantified intra-islet vessel density (Figure 4E). This failure of blood vessels to penetrate the islets transplanted into the MMP-9 knockout animals may be attributable to the extracellular matrix remodeling effects of the MMP-9 enzyme.
Newly formed blood vessels do not penetrate islets transplanted to MMP-9−/− mice. (A-D) Left panels: Confocal z-projections of image stacks (stack height 80 μm) showing transplanted wild-type islets (blue, Celltracker), vasculature (red, CD31-mAb), and Gr-1+ leukocytes (green) in cremaster muscles of wild-type (A-B) and MMP-9−/− (C-D) mice 4 days after transplantation. Bars represent 50 μm. Right panels: Single optical slices 40 μm down into the grafts illustrating the lack of intra-islet vessels in the MMP-9−/− mice, whereas the wild-type contains many newly formed capillaries. Grafts are outlined by dashed lines. Bars represent 50 μm. (E) Quantification of intra-islet vascular density in CD31-mAb–stained sections of grafts in muscle at days 3-5 after transplantation (n = 4 per day and group). *P < .05.
Newly formed blood vessels do not penetrate islets transplanted to MMP-9−/− mice. (A-D) Left panels: Confocal z-projections of image stacks (stack height 80 μm) showing transplanted wild-type islets (blue, Celltracker), vasculature (red, CD31-mAb), and Gr-1+ leukocytes (green) in cremaster muscles of wild-type (A-B) and MMP-9−/− (C-D) mice 4 days after transplantation. Bars represent 50 μm. Right panels: Single optical slices 40 μm down into the grafts illustrating the lack of intra-islet vessels in the MMP-9−/− mice, whereas the wild-type contains many newly formed capillaries. Grafts are outlined by dashed lines. Bars represent 50 μm. (E) Quantification of intra-islet vascular density in CD31-mAb–stained sections of grafts in muscle at days 3-5 after transplantation (n = 4 per day and group). *P < .05.
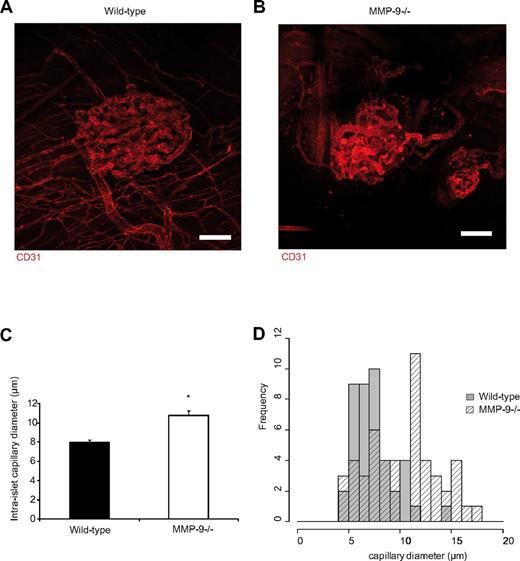
Transplanted islet vasculature is altered 2 weeks after transplantation in MMP-9−/− mice
Our experimental system leads to robust islet engraftment and allows studies of long-term effects.10 When assessing islet graft vasculature 14 days after transplantation, the islets in the wild-type and MMP-9−/− recipient mice had established a dense vascular network with intra-islet capillaries (Figure 5A-B). The vascular density was similar in both groups, but the grafts in MMP-9−/− animals displayed an altered vascular architecture because the capillaries did not form the same homogeneous vascular network and had a seemingly different tortuosity compared with grafts in wild-type recipients. The intra-islet capillaries of MMP-9−/− mice were also more heterogeneous in their vascular diameters than those of wild-type mice, and larger vessels were found within the islets transplanted into the muscle of MMP-9−/− mice, which was rarely observed in wild-type mice (Figure 5C). By quantitative histomorphic analysis, the size distribution of the intra-islet capillary diameters was different: 7.95 ± 0.32 μm in wild-type mice and 10.8 ± 0.49 μm in MMP-9−/− mice (Figure 5C-D).
Islet grafts in MMP-9−/− mice have an altered vascular phenotype at 2 weeks after transplantation. (A) Confocal z-projection of image stack showing the normal vascular network of a transplanted pancreatic islet in the cremaster muscle 14 days after transplantation. Blood vessels in red are stained with CD31-mAb. (B) Islet vasculature of an islet transplanted to the cremaster muscle of an MMP-9−/− mouse 14 days after transplantation exhibiting a disturbed vascular network with increased vessel heterogeneity. Bars represent 50 μm. (C) The intra-islet capillaries in islets transplanted to MMP-9−/− mice (n = 4 mice, 49 vessels) are on average wider than wild-type capillaries (n = 3 mice, 41 vessels). *P < .05. (D) Size-distribution histogram showing heterogeneity in capillary diameters between MMP-9−/− and wild-type mice.
Islet grafts in MMP-9−/− mice have an altered vascular phenotype at 2 weeks after transplantation. (A) Confocal z-projection of image stack showing the normal vascular network of a transplanted pancreatic islet in the cremaster muscle 14 days after transplantation. Blood vessels in red are stained with CD31-mAb. (B) Islet vasculature of an islet transplanted to the cremaster muscle of an MMP-9−/− mouse 14 days after transplantation exhibiting a disturbed vascular network with increased vessel heterogeneity. Bars represent 50 μm. (C) The intra-islet capillaries in islets transplanted to MMP-9−/− mice (n = 4 mice, 49 vessels) are on average wider than wild-type capillaries (n = 3 mice, 41 vessels). *P < .05. (D) Size-distribution histogram showing heterogeneity in capillary diameters between MMP-9−/− and wild-type mice.
Discussion
Islet transplantation has become a therapeutic option for diabetes, but improved mechanistic insights into the interactions of involved cells and molecules are needed to improve the transplantation success rates. The present study addresses 2 key questions: how do circulating leukocytes find the site of hypoxia (transplanted islet), and what are the proangiogenic mechanisms of action of these leukocytes at the site of vessel growth? These questions arose from our previous finding that recruited Gr-1+ leukocytes were crucial in the initiation of islet revascularization after transplantation into striated muscle.10 Using 3 different in vivo models, we now provide evidence that VEGF-A induces recruitment of predominantly CD11b+/Gr-1+/CXCR4hi/MMP-9hi leukocytes. Interestingly, these leukocytes expressed more CXCR4 and contained more MMP-9 than CD11b+/Gr-1+ leukocytes recruited to inflammatory stimuli, further evidencing that different neutrophil subsets exist in the circulation and may be selectively recruited to different situations.
Recently, we found that transplanted syngeneic islets of Langerhans attract leukocytes to a high degree.10 This brought forth the hypothesis that the grafts produce a soluble agent that attracts leukocytes from nearby blood vessels. VEGF-A is a potent endothelial cell mitotic agent41 whose expression is controlled by the hypoxia-inducible factor system, leading to increased expression at low tissue oxygenation.42 β-Cells in pancreatic islets secrete high levels of VEGF-A29,31 required for establishment of the specialized, fenestrated, dense, and tortuous capillary network of these miniature endocrine organs.31 VEGF-A has been reported to induce expression of SDF-1α/CXCL12 in parallel with myeloid cell homing to tissues of high expression,43 but its direct properties on immune cell recruitment in vivo are fairly unknown. In the present study, the role of islet-secreted VEGF-A and its effect on leukocyte recruitment and islet revascularization were addressed by transplanting islets that are deficient in β-cell VEGF-A (pdx1-cre; VEGFfl/fl) to wild-type mice. Thus, only the transplanted islets were incapable of secreting VEGF-A in the otherwise normal animal. In the days after transplantation, wild-type islets secreting VEGF-A had more than twice the amount of surrounding leukocytes than the VEGF-A–deficient islets. This pattern is in line with what has been seen in other conditional models, in which turned-on VEGF-A expression leads to high myeloid cell recruitment and retention,24,43 and a switched-off expression during neovascularization leads to low leukocyte recruitment and vascularity.24 To study the kinetics of VEGF-A–induced leukocyte recruitment, intravital microscopy of the cremasteric microcirculation during addition of VEGF-A to the superperfusion buffer was performed. These experiments revealed that VEGF-A, mostly referred to as an angiogenic molecule released to induce endothelial cell migration and sprouting in hypoxic tissue, is also capable of acutely inducing leukocyte adhesion to postcapillary venules, but not arterioles, followed by emigration into tissue. This effect was observed already after 15 minutes (data not shown), which indicates that this is either a direct VEGF-A effect on leukocytes in circulation or caused by a release of prestored agents involved in recruitment (adhesion molecules, chemokines) because de novo protein synthesis require more time but might be induced in parallel.
Up-regulation of adhesion molecules (ICAM-1, VCAM-1, and E-selectin) on human umbilical vein endothelial cells was detected after 2-4 hours of VEGF-A exposure in vitro.44 In the present study, we found increased leukocyte recruitment as early as 15-30 minutes after addition of VEGF-A to the superperfusate, and it therefore seems unlikely that the observed fast effect of VEGF-A on leukocyte recruitment is the result of transcriptional up-regulation of endothelial adhesion molecules. A recent study demonstrated an activation by VEGF-A and SDF-1α of myeloid cell PI3Kγ leading to integrin α4β1 activation and subsequent leukocyte invasion of tumors.28 The in vivo kinetics of such activation mechanism in our system and the relevance of this signaling for CD11b+/Gr-1+/CXCR4hi/MMP-9hi neutrophils in angiogenesis are interesting topics for future research.
In addition to inducing adhesion molecules, VEGF-A has been shown to induce chemokines, such as SDF-1α/CXCL12,43 which has effects on leukocytosis and progenitor cell mobilization.20-23 However, levels of SDF-1α did not increase by VEGF-A-superperfusion in our experimental settings. It remains to be determined whether local release and presentation of SDF-1α or other chemotactic agents are affected. To the best of our knowledge, there are no published reports of instant chemokine release in response to VEGF-A. Further, VEGF-A was first named vascular permeability factor on the basis of its potent effect on vascular permeability through opening of the endothelial junctions. The impact of increased permeability for leukocyte transmigration has been debated, but a recent study convincingly shows that these events are not linked.45 We have confirmed this in our models (S.M. and M.P., unpublished data, June 2012). Thus, the permeability-increasing effect of VEGF-A by itself would most probably not induce leukocyte recruitment to tissue.
The leukocytes recruited to VEGF-A were characterized by flow cytometric phenotype analysis and compared with those recruited to MIP-2. The results showed that VEGF-A was similar to MIP-2 in recruiting CD11b+/Gr-1+/MMP-9+ neutrophils, a cell type involved in both pathogen removal46 and angiogenesis.5,10,14,47 The neutrophils were also analyzed for CXCR4 expression, the receptor for SDF-1α/CXCL12, which is involved in neutrophil mobilization from bone marrow and homing to angiogenic tissue.23 CXCR4 has been shown to be expressed by tumor-infiltrating neutrophils.48 We found that the leukocytes present at islet grafts shortly after transplantation (4 days) were CD11b+/Gr-1+ cells that expressed high levels of CXCR4. Interestingly, the leukocytes that were recruited to intraperitoneal VEGF-A expressed more CXCR4 than the cells recruited to the inflammatory stimulus.
Flow cytometric analysis is not ideal to assess differences in MMP-9 levels of the recruited cells. When the MMP-9 levels of the different cell isolates were investigated by gelatin zymography, we found that neutrophils recruited by VEGF-A exerted higher MMP-9 levels compared with cells recruited to MIP-2. There is emerging evidence for subset division of neutrophils,11,49 and our data further support this by suggesting that there may be a subset of neutrophils more primed and prone to respond to a hypoxic stimulus, expressing more CXCR4 and containing more MMP-9.7 There is also the possibility of de novo synthesis of both CXCR4 and MMP-9 by peritoneal leukocytes after being subjected to the VEGF-A stimulus. However, when the possibility of changing leukocyte expression of CXCR4 was investigated by stimulating the cells in vitro with MIP-2 or VEGF-A for 30 minutes, neither leukocytes isolated from blood nor intraperitoneally recruited cells shifted their expression of surface markers, further supporting the notion of preexisting proangiogenic leukocyte subsets.
Ultimately, we tested the effects of the enzyme MMP-9 in a clinically relevant model of islet transplantation where MMP-9–deficient mice received isolated pancreatic islets into striated muscle. Compared with islets transplanted into wild-type mice, grafts in MMP-9−/− mice had significantly fewer intra-islet capillaries 3-5 days after transplantation, despite similar levels of recruited CD11b+/Gr-1+ neutrophils. Although MMP-9−/− mice do not display a spontaneously apparent general vascular phenotype in the adult,50 the lack of MMP-9 does result in phenotypic differences in tumor angiogenesis,19 and in revascularization of hypoxic islets, as demonstrated in this study. At 14 days after transplantation, there were no longer clear differences in intra-islet vascular density, indicating that revascularized islets may have overcome the lack of MMP-9 by compensatory mechanisms, perhaps by other matrix metalloproteinases (eg, MMP-2 or MMP-3). However, when carefully studying these islets by confocal microscopy, qualitative differences of the newly formed vessels were found compared with what was observed in the wild-type mice. The islet vasculature was more heterogeneous in size and tortuosity, indicating also a long-term role for MMP-9 in vascular remodeling after islet transplantation and revascularization. Whether this difference in the vasculature has an effect on islet function is, however, not yet clear. A unique feature of neutrophils is that they produce and release MMP-9 without its endogenous inhibitor tissue inhibitor of metalloproteinases-1. Thus, neutrophil-delivered MMP-9 is highly active when secreted in tissue and may exert many different proangiogenic actions. For instance, matrix digestion not only facilitates vessel tunneling during angiogenesis, but also liberates growth factors bound to tissue stroma reported to be important during angiogenesis.6,15,16
In this study, we found that VEGF-A, released in high amounts during hypoxia, was capable of recruiting leukocytes, demonstrating a new role for this protein during hypoxia. Further, the most predominant leukocyte type recruited by VEGF-A was a CD11b+/Gr-1+/CXCR4hi proangiogenic subset carrying high amounts of an angiogenic effector protein, MMP-9. This enzyme was shown to be important in the revascularization of transplanted pancreatic islets as mice lacking MMP-9 exhibited altered revascularization. Taken together, this illustrates that circulating CXCR4hi neutrophils are recruited to hypoxic sites by VEGF-A, and these cells release MMP-9 to facilitate rapid angiogenesis and restoration of perfusion. The identified cells and molecules have consequences for the clinical management of islet transplantation and call for careful use of drugs that inhibit VEGF-A and MMP-9 or that influence recruitment of angiogenic leukocytes.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anders Ahlander and Antoine Giraud (Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden) for skilled technical assistance and Greet Thijs (Rega Institute for Medical Research, University of Leuven, Leuven, Belgium) for excellent care for the backcrosses and maintenance of the MMP-9−/− colonies.
All experiments were done in the laboratory of M.P. or connected core facilities, except gelatin zymography assays, which were performed in the laboratory of G.O.
This work was supported by the Swedish Research Council (K2012-99x), the Swedish Diabetes Association, the Clas Groschinsky Foundation, the Jeansson Foundation, the Thuring Foundation, the Family Ernfors Foundation, the Swedish Diabetes Foundation, the Belgian Geconcerteerde Onderzoeksacties (GOA2012/017), and the Fund for Scientific Research of Flanders (FWO-Vlaanderen), and supported in part by the National Institutes of Health (grants DK69603, DK089572, and DK63439), the Veterans Administration Research Service (Merit Review Award), the Juvenile Diabetes Research Foundation International, the Vanderbilt Mouse Metabolic Phenotyping Center (National Institutes of Health grant DK59637), and the Vanderbilt Diabetes Research and Training Center (National Institutes of Health grant DK20593).
National Institutes of Health
Authorship
Contribution: G.C. designed experiments, performed research, analyzed data, and wrote the manuscript; E.V. designed experiments, performed research, and analyzed data; J.V., M.L., and S.M. performed research and analyzed data; R.B.R., M.B., and A.C.P. contributed with mice and designed experiments; G.O. contributed with mice, designed experiments, and wrote the manuscript; and M.P. initiated the study, designed experiments, wrote the manuscript, and supervised the research project.
Conflict-of-interest disclosure: A.C.P. has received an investigator-initiated research grant from Eli Lilly and Company. The remaining authors declare no competing financial interests.
Correspondence: Mia Phillipson, Department of Medical Cell Biology, Uppsala University, PO Box 571, 75123 Uppsala, Sweden; e-mail: mia.phillipson@mcb.uu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal