Abstract
Targeted and immune-based therapies are thought to eradicate cancer cells by different mechanisms, and these approaches could possibly complement each other when used in combination. In this study, we report that the in vivo antitumor effects of the c-KIT inhibitor, dasatinib, on the c-KIT mutant P815 mastocytoma tumor were substantially dependent on T cell–mediated immunity. We found that dasatinib treatment significantly decreased levels of Tregs while specifically enhancing tumor antigen-specific T-cell responses. We sought to further enhance this therapy with the addition of anti-OX40 antibody, which is known to provide a potent costimulatory signal to T cells. The combination of dasatinib and anti-OX40 antibody resulted in substantially better therapeutic efficacy compared with either drug alone, and this was associated with enhanced accumulation of tumor antigen-specific T cells in the tumor microenvironment. Furthermore, the combination regimen inhibited the function of Tregs and also resulted in significantly up-regulated expression of the IFN-γ–induced chemokines CXCL9, 10, and 11 in the tumor microenvironment, which provides a feasible mechanism for the enhanced intratumoral CTL infiltration. These studies delineate a strategy by which targeted therapy and immunotherapy may be combined to achieve superior antitumor responses in cancer patients.
Introduction
Molecular-targeted therapy inhibits the growth of cancer cells by blocking the activity of specific oncogenic signaling molecules which drive the growth of tumors. Existing targeted therapy drugs can induce transient regression of large tumors, but the complexity and instability of the cancer genome pose a significant challenge to targeted therapy and recurrence with drug-resistant tumor variants is a common problem.1-3 One such clinical example is c-KIT, a proto-oncogenic tyrosine kinase receptor. Genetic aberrations of this gene have been shown to be related to the incidence of several types of cancers, including gastrointestinal stromal tumor (GIST), melanoma, mast cell leukemia, and germ cell tumors.4,5 Treating GIST with c-KIT inhibitors has dramatically changed patient prognosis, but most patients will still eventually relapse because of the emergence of secondary mutations and drug-resistant tumor clones.6 For melanoma, dramatic responses have been seen in some patients with c-KIT mutations, but the overall efficacy and duration of c-KIT inhibitors have been disappointing.7-9 Similarly, results from clinical trials with c-KIT inhibitors in systemic mastocytosis patients have also been unsatisfactory.10-12 The heterogeneous nature of cancer is the main barrier for optimal therapeutic efficacy and suggests that combination therapies may have potential therapeutic promise for treating cancers that do not respond well to single-agent therapies.
The goal of immunotherapy is to eliminate cancer cells by boosting the antitumor immune response in the body. The effectiveness of immunotherapy has been demonstrated in several malignancies, such as melanoma, renal carcinoma, and lymphoma.13-19 Although overall response rates remain relatively modest, durable and complete responses are observed in some patients. Because targeted therapy and immunotherapy eliminate tumor cells by distinct mechanisms and have complementary strengths and weaknesses, combining these 2 types of therapies for cancer treatment is particularly attractive.
Deep-sequencing analysis of DNA from several different cancers has shown that tumor cells accumulate multiple mutations that can potentially serve as antigenic targets for the adaptive immune system,20 suggesting that the antitumor immune response might be enhanced on apoptosis of tumor cells. Studies showing that the effectiveness of some chemotherapy regimens requires an intact immune system further support this notion.21-24 Although the therapeutic effects of targeted therapies are believed to rely on the direct inhibition of oncogenic proteins, we wanted to determine whether the underlying immune response also contributes to the antitumor effects observed. And we set out to test the hypothesis that the efficacy of a targeted therapy drug could be augmented by immune-boosting adjuvants.
However, it has been challenging to study the combination of targeted therapy drugs and immunotherapy agents in preclinical models because these studies need to be done in immunocompetent animals and few murine tumors are both immunogenic and driven by a single oncogenic event. We chose to use the P815 mastocytoma model because this tumor is driven by a drug-targetable–activating mutation (D814Y) in the c-KIT receptor and its antitumor immune responses have been well studied. P815 tumors elicit measurable T cell–mediated antitumor immunity, and 2 tumor antigens (P1A and P1E) in this model have been well characterized.25,26 Dasatinib, a small molecule tyrosine kinase inhibitor approved by the Food and Drug Administration for the treatment of CML and Ph+AML, has been shown to potently inhibit D814Y mutant c-KIT in P815 and a human mastocytoma cell line, inducing growth arrest and apoptosis of these tumor cells in vitro.27-29 In addition to its effects on the D814Y mutant c-KIT, this drug has a very short biologic half-life (3-6 hours)27 We consider the short half-life as an advantage for being combined with immune-boosting agents because most of the c-KIT tyrosine kinase inhibitors have been shown to negatively interfere with T-cell proliferation and function.30-32
Our results show that the therapeutic effect of dasatinib on P815 mastocytoma in vivo is crucially dependent on the presence of a CD8+ T cell–mediated antitumor immune response, suggesting that the development of antitumor immunity is an underlying contributory factor to the therapeutic effect of this targeted therapy. Furthermore, we found that the therapeutic effect of dasatinib could be significantly boosted when used in combination with an antibody against OX40, a T-cell costimulatory molecule. These results delineate a complementary mechanism by which targeted and immune therapies can be combined to improve antitumor efficacy, and suggest that clinical trials using this combination strategy may be warranted.
Methods
Cell lines, reagents, and animals
P815 murine mastocytoma cells were obtained from ATCC. All tumor cell lines were maintained in MEM with 10% FBS and 100 μg/mL Normocin (Invivogen). DBA/2 mice, 6-10 weeks old, were purchased from National Cancer Institute (Bethesda, MD) and maintained in a pathogen-free animal facility at MD Anderson Cancer Center. P1CTL transgenic mice have been described.33 Mice were handled in accordance with protocols approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee. Dasatinib was purchased from LC Laboratories and dissolved in vehicle (50% polyethylene glycol, 50% H2O) before gavage. Anti-OX40 antibody was purchased from Harlan. CD8 depletion antibody (clone HB129/116-13.1), CD4 depletion antibody (clone GK1.5), and rat IgG 2b isotype control antibody were purchased from Bioxcell.
FACS reagents and staining
FACS analysis was performed using the following antibodies: αCD16/CD32, FITC-conjugated αCD3, allophycocyanin (APC)–conjugated αCD8, PE-conjugated αIFN-γ (purchased from BD Biosciences); APC-conjugated αFoxp3, PerCP-Cy5.5–conjugated αCD25, PE-conjugated αCD4, FITC-conjugated αCD8, Pacific Blue–conjugated αCD8 (purchased from eBioscience); PE-conjugated P1A tetramer (synthesized at the MHC Tetramer Production Facility of Baylor College of Medicine, Houston, TX). IFN-γ and Treg staining was performed according to the protocol provided by the manufacturers (BD Biosciences and eBioscience). Cells were analyzed using the Caton II flow cytometer, with data analyzed by using Diva software (BD Biosciences).
Tumor treatment and monitoring
DBA/2 mice were subcutaneously inoculated with 2 × 106 P815 tumor cells on the left abdominal wall on day 0. Mice with moderate tumor size were selected and divided into different groups on day 8 (6-10 mice in each group). Tumor-bearing mice were treated with vehicle or dasatinib (150 mg/kg) on days 8, 9, and 10 by gavage. In the combination treatment experiments, mice were also injected intraperitoneally with anti-OX40 antibody 200 μg/mice on days 10 and 13. Tumor growth was monitored by measuring the perpendicular diameters of tumors. Mice were killed when the tumor diameters exceeded 20 mm. For CD4+/CD8+ T-cell depletion experiments, tumor-bearing mice were injected intraperitoneally with 200 μg of monoclonal antibodies starting from day 8 once a week. Rat IgG was used as control antibody. Depletion of CD4+ and CD8+ T cells was confirmed by analyzing the peripheral blood of the mice by flow cytometry
Monitoring tumor antigen-specific T-cell response
On days 14, 18, 21, and 26, 50 μL of peripheral blood was collected by using tail-clip method and then treated with ACK lysis buffer (Cambrex) for 5 minutes to deplete the red blood cell. Cells were stained with αCD3, αCD8, and P1A tetramer antibodies. For IFN-γ intracellular staining, cells were stimulated with or without 10 μg/mL P1A or P1E peptides in the presence of 1:1000 Golgiplug (BD Biosciences) for 6 hours. Cells were then collected and stained with αCD3, αCD8, and αIFN-γ antibodies. The percentage of antigen-specific T cells was analyzed by using flow cytometry. In another experiment, collected peripheral blood cells were stimulated with or without P1A or P1E peptide for 24 hours. P1A and P1E peptides were purchased from Peptides International. The supernatants were harvested and IFN-γ was detected by ELISA. The IFN-γ ELISA kit was purchased from Biolegend.
Dendritic cell vaccination
Murine bone marrow cells were grown in complete medium with 100 ng/mL GM-CSF for 8 days, then stimulated with 1 μg/mL LPS for 1 day. Nonadherent cells were collected and resuspended in Opti-MEM medium at a concentration of 2 × 106 cells/mL, and pulsed with 10 μg/mL P1A peptide for 4 hours at 37°C. On day −3, 3 million splenocytes from P1CTL transgenic mice were transferred into DBA/2 mice via IV injection. Then, mice were treated with vehicle or dasatinib for 3 days. On day 0, 1 million P1A peptide-pulsed DCs were transferred into DBA/2 mice. Peripheral blood was collected on day 7 for flow cytometric analysis.
Immunohistochemistry
Harvested tumors were embedded in OCT and snap-frozen in liquid nitrogen. Immunohistochemistry was done by the Research Histopathology Facility at MD Anderson Cancer Center.
Real-time PCR
Total RNA was isolated from tumors on day 16 after tumor inoculation by using the RNAeasy Micro Kit (QIAGEN) according to the protocol provided by the manufacturer. The panel of chemokine primers was synthesized by Invitrogen. The chemokines on the real-time PCR panel include CCL1-13, CCL17, CCL19-22, CCL24-25, CCL27-28, CXCL1-2, CXCL4-5, CXCL7, CXCL9-16, XCL1, and CX3CL1. GAPDH was used as the normalization reference gene. SYBR Green real-time PCR was done by using Bio-Rad C1000 thermal cycler. Real-time PCR data analysis was performed using Bio-Rad CFX manager software.
Harvest of tumor-infiltrating lymphocytes
On day 16, tumors were harvested and dissected into fragments by cutting, and then digested in tumor digestion buffer for 2 hours at 37°C. The tumor digestion buffer was made by dissolving 1 mg/mL collagenase, 100 μg/mL hyaluronidase, and 20 mg/mL Dnase (Sigma-Aldrich) in RPMI medium. Then, tumor digest was filtered through 70-μm nylon mesh. Cell suspension was layered over the Lympholyte-M density separation medium (Cedarlane) and centrifuged at 1000g for 20 minutes. Lymphocyte layer at the interface was isolated and analyzed by flow cytometry.
Treg suppression assay
Mice were treated with 3 doses of vehicle or dasatinib (150 mg/kg) and one dose of anti-OX40 (200 μg/mice). Splenocytes of mice were collected and stained with αCD4, αCD8, αCD25 antibodies. CD4+ CD25high cells from the different groups were sorted as Treg cells by flow cytometry, and CD8+ cells were sorted as effector T cells. Effector T cells were labeled with 1μM CFSE for 5 minutes at 37°C. Sorted Treg cells and effector T cells were cocultured at a 2:1 ratio in 96-well round-bottom plate with non-T-cell splenocytes as APCs, 30 IU/mL IL-2 and 0.1 μg/mL α CD3. Three days later, cells were analyzed for CFSE dilution by flow cytometric analysis.
Statistical analysis
Data are represented as mean ± SEM. The statistical differences between different groups were determined by the Student t test or 1-way ANOVA test in GraphPad Prism software. Mouse survival between groups was compared by using the log-rank test in GraphPad Prism software. All results shown in the manuscript are representative of at least 3 independent experiments with similar results.
Results
T cell–mediated immunity contributes to the antitumor effects of dasatinib
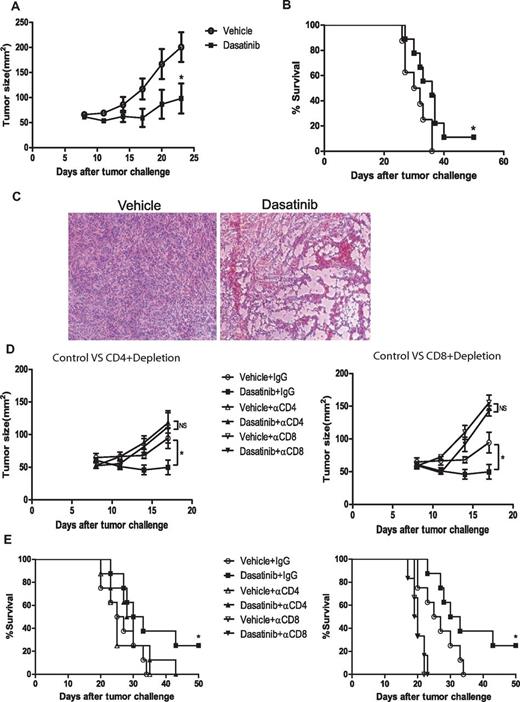
Because dasatinib was previously shown to block the activity of D814Y mutant c-kit receptor in P815 mastocytoma leading to apoptosis in vitro,28,29 we first set out to assess the antitumor activity of dasatinib against P815 tumor in vivo. We treated the tumor-bearing mice with daily gavage of vehicle or dasatinib (150 mg/kg) on days 8, 9, and 10 following tumor inoculation. Dasatinib treatment significantly decreased the tumor volumes and prolonged mouse survival (Figure 1A-B). H&E staining revealed extensive vacuolar degeneration in dasatinib-treated tumors, morphologic changes consistent with significant tumor cell death (Figure 1C).
In vivo antitumor effect of dasatinib on P815 mastocytoma. DBA/2 mice were subcutaneously inoculated with 2 × 106 P815 tumor cells on day 0. Tumor-bearing mice were treated with vehicle or dasatinib (150 mg/kg) on day 8, 9, and 10 by gavage. For depletion experiment, mice were injected IP with 200 μg of rat IgG or CD8 depletion antibodies starting from day 8 once a week. (A) Tumor sizes of mice treated with vehicle or dasatinib (*P = .0354). (B) Kaplan-Meier survival curve of the tumor-bearing mice for 2 different groups (*P = .0447). (C) Representative H&E-stained slides of control tumor and dasatinib-treated tumor. (D) Tumor sizes of CD4+/CD8+ T-cell depletion experiment (vehicle + IgG vs dasatinib + IgG: *P = .0427). (E) Kaplan-Meier survival curve of CD4+/CD8+ T-cell depletion experiment (vehicle + IgG vs dasatinib + IgG: *P = .0503).
In vivo antitumor effect of dasatinib on P815 mastocytoma. DBA/2 mice were subcutaneously inoculated with 2 × 106 P815 tumor cells on day 0. Tumor-bearing mice were treated with vehicle or dasatinib (150 mg/kg) on day 8, 9, and 10 by gavage. For depletion experiment, mice were injected IP with 200 μg of rat IgG or CD8 depletion antibodies starting from day 8 once a week. (A) Tumor sizes of mice treated with vehicle or dasatinib (*P = .0354). (B) Kaplan-Meier survival curve of the tumor-bearing mice for 2 different groups (*P = .0447). (C) Representative H&E-stained slides of control tumor and dasatinib-treated tumor. (D) Tumor sizes of CD4+/CD8+ T-cell depletion experiment (vehicle + IgG vs dasatinib + IgG: *P = .0427). (E) Kaplan-Meier survival curve of CD4+/CD8+ T-cell depletion experiment (vehicle + IgG vs dasatinib + IgG: *P = .0503).
To assess a potential role for T cell–mediated immunity in the therapeutic efficacy of dasatinib, we depleted CD4+ and CD8+ T cells in the mice before treatment. Interestingly, both CD4+ T-cell depletion and CD8+ T-cell depletion reduced antitumor responses in dasatinib-treated mice almost to the same level as in vehicle-treated mice, effectively abrogating the survival benefit provided by dasatinib (Figure 1D-E). This data revealed that an underlying immune response contributes substantially to the therapeutic antitumor effect of dasatinib in this model.
Dasatinib can increase levels of circulating tumor antigen-specific T cells
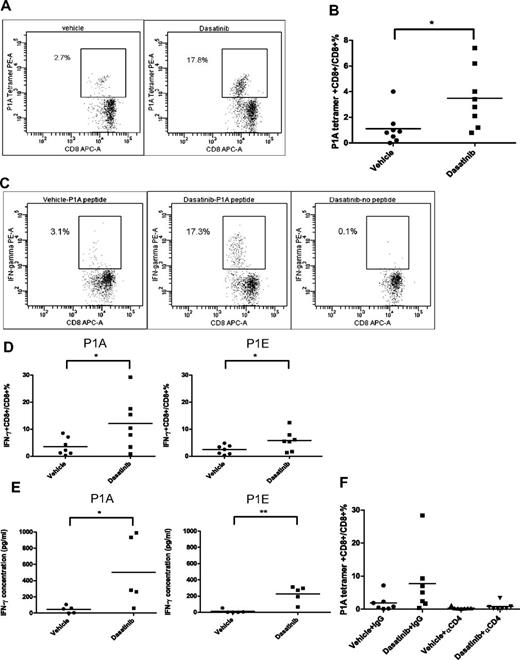
We next sought to determine the effect of dasatinib on the tumor antigen-specific T-cell response. P815 is an immunogenic tumor which can elicit spontaneous T-cell priming against tumor antigens. Using a P1A-specific tetramer and flow cytometry, we found that 3 days of dasatinib treatment significantly increased the tumor antigen-specific T-cell levels in the peripheral blood of tumor-bearing mice (Figure 2A-B). Peripheral blood T cells were also examined for antigen-specific IFN-γ production using intracellular staining following stimulation with or without P1A, P1E peptides for 6 hours. We found that while CD8+ T cells from vehicle-treated mice were relatively unresponsive to peptide stimulation, a significant proportion of CD8+ T cells from dasatinib-treated mice showed IFN-γ positivity (Figure 2C-D). IFN-γ ELISA showed results consistent with the intracellular IFN-γ staining, which indicated that 3 days of dasatinib treatment significantly augmented the antitumor T-cell response (Figure 2E). Moreover, CD4+ T-cell depletion in vivo significantly compromised the enhanced tumor antigen-specific CD8+ T-cell response mediated by dasatinib (Figure 2F), suggesting that the CD8+ T-cell response against P815 is T helper cell–dependent. Dasatinib is a multiple tyrosine kinase inhibitor which inhibits the activities of c-KIT, EphA, Src family members, Brc/abl, PDGFR, and some other tyrosine kinases.27 It has been reported that dasatinib inhibits TCR signal transduction, cellular proliferation, and cytokine production by T cells on antigen stimulation probably because of the inhibitory effect of dasatinib on Src family member Lck.34,35 Because we observed only enhanced T-cell responses using a 3-day dasatinib regimen, next we investigated whether increasing the length of time of dasatinib treatment is harmful or helpful to its therapeutic efficacy on P815. We found that prolonged dasatinib treatment did not provide any enhanced antitumor effects or survival benefit compared with short-term dasatinib treatment (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In fact, P1A tetramer staining showed that long-term dasatinib treatment actually compromised the positive effect of dasatinib on tumor antigen-specific CD8+ T cell priming (supplemental Figure 1C). Our data together with that of other groups suggests that antigen-specific T-cell responses can be impaired with the persistent presence of tyrosine kinase inhibitor. But our findings also indicate that dasatinib is capable of enhancing antitumor immune responses if the dosing and schedule are optimized.
Effect of dasatinib treatment on tumor antigen-specific T-cell response. (A) Representative dot plots for P1A tetramer staining in the periphery blood of vehicle- or dasatinib-treated mice. (B) Percentages of P1A tetramer+ T cells in total CD8+ T cells (*P = .0235). (C) Representative dot plots for intracellular IFN-γ staining. (D) Percentages of IFN-γ+ T cells in total CD8+ T cells (P1A: *P = .0449, P1E: *P = .0498). (E) IFN-γ secretion by peripheral blood T cells in response to P1A or P1E peptides detected by ELISA (P1A: *P = .0428, P1E: **P = .0017). (F) P1A tetramer staining of the CD4+ T-cell depletion experiment.
Effect of dasatinib treatment on tumor antigen-specific T-cell response. (A) Representative dot plots for P1A tetramer staining in the periphery blood of vehicle- or dasatinib-treated mice. (B) Percentages of P1A tetramer+ T cells in total CD8+ T cells (*P = .0235). (C) Representative dot plots for intracellular IFN-γ staining. (D) Percentages of IFN-γ+ T cells in total CD8+ T cells (P1A: *P = .0449, P1E: *P = .0498). (E) IFN-γ secretion by peripheral blood T cells in response to P1A or P1E peptides detected by ELISA (P1A: *P = .0428, P1E: **P = .0017). (F) P1A tetramer staining of the CD4+ T-cell depletion experiment.
Dasatinib treatment can decrease the proportion of circulating regulatory T cells and enhance vaccine-mediated T-cell priming
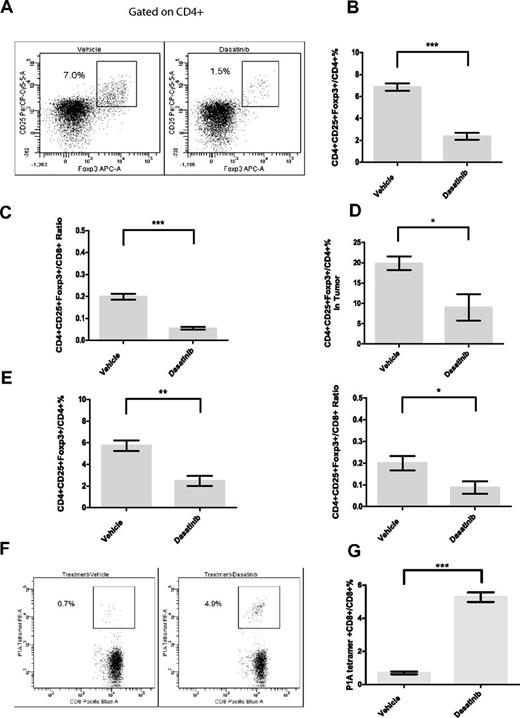
Dasatinib has been reported to suppress the proliferation and function of Tregs in vitro,36 but the in vivo effect of dasatinib treatment on murine Treg cells has not been reported. Flow cytometric analysis showed that dasatinib alone significantly decreased the percentage of Foxp3+CD25+ Treg cells in total CD4+ T cells in the peripheral blood of tumor-bearing mice (Figure 3A-B). The ratio of Tregs to CD8+ effector T cells was also markedly decreased by dasatinib (Figure 3C). We also examined the Treg levels within the tumor microenvironment immediately following dasatinib treatment and found a similar decrease in cell numbers (Figure 3D). However, this decrease was transient, as Treg cell numbers went back to normal 3 days after drug withdrawal (supplemental Figure 2). Because the drop in Tregs was significant, we next investigated whether the short Treg-depleted window caused by dasatinib can render T cells more amenable to vaccine-induced antigen-specific expansion in a tumor-free system. In tumor-free mice, dasatinib caused a similar decrease in Treg cells (Figure 3E). To assess whether dasatinib could enhance vaccine-mediated priming, we IV injected the mice with splenocytes from P1CTL transgenic mice, whose TCRs are specific for P1A peptide/H2Ld complexes. After 3 days of dasatinib treatment, P1A peptide-pulsed dendritic cells were transferred into the recipients. Flow cytometric analysis of peripheral blood on day 7 after vaccination showed that P1A-specific T-cell levels were significantly higher in dasatinib-treated mice (Figure 3F-G). Therefore, this result supports the idea that dasatinib preferentially decreases Tregs in vivo and makes the host immune environment more favorable for antigen-driven effector T-cell proliferation.
Effect of 3-day dasatinib treatment on levels of regulatory T cells and vaccine-mediated antigen-specific CD8+ T-cell responses. (A) Representative dot plots for Treg staining in the peripheral blood of tumor-bearing mice. (B) Percentages of Treg cells in CD4+ T cells in the peripheral blood of tumor-bearing mice on day 11 (***P < .0001). (C) Treg/ T effector ratio in the peripheral blood of tumor-bearing mice on day 11 (***P < .0001). (D) Percentages of Treg cells in CD4+ T cells in tumors on day 11 (*P = .0473). (E) Effect of dasatinib on proportions of Treg cells and Treg/T effector ratios in tumor-free mice (**P = .0026, *P = .0432). (F) Representative dot plots for P1A tetramer staining in the peripheral blood on day 7 after vaccination. (G) Effect of 3-day dasatinib treatment on vaccine-mediated P1A-specific CD8+ T-cell responses (***P < .0001).
Effect of 3-day dasatinib treatment on levels of regulatory T cells and vaccine-mediated antigen-specific CD8+ T-cell responses. (A) Representative dot plots for Treg staining in the peripheral blood of tumor-bearing mice. (B) Percentages of Treg cells in CD4+ T cells in the peripheral blood of tumor-bearing mice on day 11 (***P < .0001). (C) Treg/ T effector ratio in the peripheral blood of tumor-bearing mice on day 11 (***P < .0001). (D) Percentages of Treg cells in CD4+ T cells in tumors on day 11 (*P = .0473). (E) Effect of dasatinib on proportions of Treg cells and Treg/T effector ratios in tumor-free mice (**P = .0026, *P = .0432). (F) Representative dot plots for P1A tetramer staining in the peripheral blood on day 7 after vaccination. (G) Effect of 3-day dasatinib treatment on vaccine-mediated P1A-specific CD8+ T-cell responses (***P < .0001).
Addition of anti-OX40 antibody improves the antitumor efficacy of dasatinib treatment
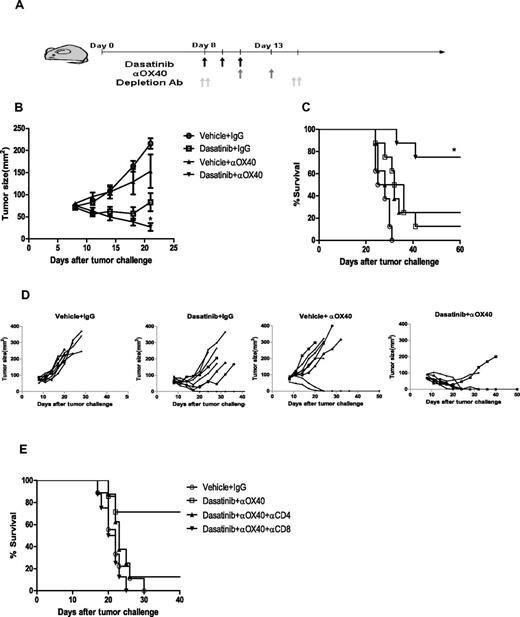
Because we found that the antitumor effects of dasatinib were largely dependent on CD8+ T cells, we hypothesized that providing further costimulation of these T cells with an agonistic antibody against OX40, could further boost the therapeutic response. OX40 is a costimulatory molecule belonging to the TNF receptor family and is known to play an important role in the differentiation, expansion, survival, and migration of T cells.37,38 Furthermore, anti-OX40 has been shown to potentiate antitumor immune responses in multiple in vivo tumor models.39,40 We treated 8-day tumor-bearing mice with dasatinib or vehicle for 3 days and then administered 2 doses of anti-OX40 antibody on days 10 and 13 (Figure 4A). This combination regimen lead to significantly improved tumor regression and complete cures in 75% of the mice (Figure 4B-D). Neither dasatinib or anti-OX40 alone induced similar regression or survival, suggesting therapeutic synergy of the 2 treatments. The therapeutic effect of the combination treatment was striking considering the large tumor burden (60 mm2-80 mm2) at the time of treatment. Depletion of CD8+, and to a lesser extent CD4+, T cells abolished the therapeutic effect of dasatinib combined with anti-OX40 (Figure 4E), supporting the notion that T cells are the major immune effector cells responsible for the therapeutic effect of this combination regimen.
Therapeutic effect of combining dasatinib and anti-OX40 antibody. Mice received daily gavage of vehicle or dasatinib (150 mg/kg) on day 8-10 and IP injection of IgG control antibody or anti-OX40 antibody (200 μg/mice) on day 10 and 13. (A) Schema of treatment. (B) Tumor sizes of mice for 4 different groups (vehicle + IgG vs dasatinib + anti-OX40: ***P < .0001, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0042, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0257). (C) Kaplan-Meier survival curve of the tumor-bearing mice for 3 different groups (vehicle+IgG vs dasatinib + anti-OX40: ***P < .0001, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0074, vehicle + anti-OX40 versus dasatinib + anti-OX40: *P = .0151). (D) Plots represent the tumor size of individual mice for each group. (E) Kaplan-Meier survival curve of the CD4+/CD8+ T-cell depletion experiment.
Therapeutic effect of combining dasatinib and anti-OX40 antibody. Mice received daily gavage of vehicle or dasatinib (150 mg/kg) on day 8-10 and IP injection of IgG control antibody or anti-OX40 antibody (200 μg/mice) on day 10 and 13. (A) Schema of treatment. (B) Tumor sizes of mice for 4 different groups (vehicle + IgG vs dasatinib + anti-OX40: ***P < .0001, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0042, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0257). (C) Kaplan-Meier survival curve of the tumor-bearing mice for 3 different groups (vehicle+IgG vs dasatinib + anti-OX40: ***P < .0001, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0074, vehicle + anti-OX40 versus dasatinib + anti-OX40: *P = .0151). (D) Plots represent the tumor size of individual mice for each group. (E) Kaplan-Meier survival curve of the CD4+/CD8+ T-cell depletion experiment.
The combination regimen increases the infiltration of tumor-specific effector T cells in the tumor microenvironment
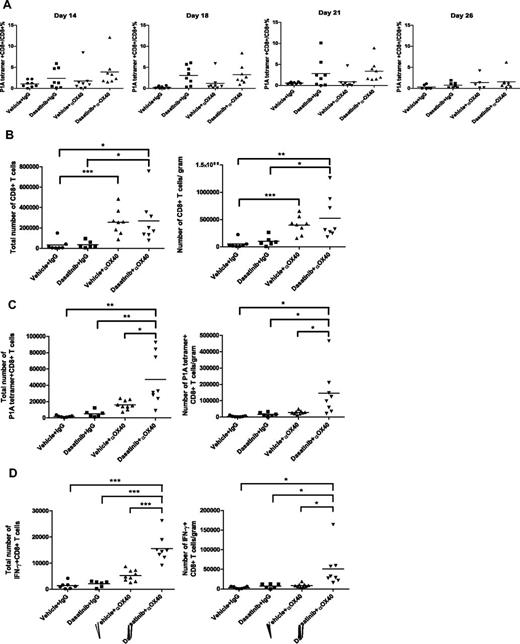
Although it has been reported that OX40 agonist therapy can enhance the expansion and survival of CTL, we found that addition of anti-OX40 to the dasatinib regimen did not further enhance or prolong the tumor antigen-specific T-cell response in our tumor model: the percentages of P1A tetramer+CD8+ T cells were similar between the dasatinib-treated group and the combination-treated group (Figure 5A). Considering that the combination treatment yielded a much better therapeutic effect than dasatinib alone, we next sought to assess the effect of this combination regimen on T-cell infiltration of the tumor site. Thus, tumor infiltrating lymphocytes were isolated from P815 tumors on day 18 and subjected to flow cytometric analysis. We found that anti-OX40 alone increased the overall infiltration of CD8+ effector T cells, as previously reported. And the combination of dasatinib and anti-OX40 led to enhanced infiltration of P1A-specific T cells into the tumors, to levels greater than that observed with either anti-OX40 or dasatinib alone (Figure 5B-C). Consistent with the tetramer analysis, mice receiving the combination demonstrated the highest levels of IFN-γ production in response to P1A antigen stimulation (Figure 5D).
Influence of dasatinib and anti-OX40 treatment on the tumor-infiltrating lymphocytes. (A) Effect of dasatinib and anti-OX40 on levels of the P1A-specific T cells in the blood. (B) Total numbers of CD8+ T cells and the ratios of CD8+ T-cell number/tumor weight (for CD8+ T-cell total number, vehicle + IgG vs vehicle + anti-OX40: ***P = .0007, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0250, vehicle + IgG vs dasatinib + anti-OX40: *P = .0165; for CD8+ T-cell number/gram, vehicle + IgG vs vehicle + anti-OX40: ***P = .0002, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0234, vehicle + IgG vs dasatinib + anti-OX40: **P = .0076). (C) Total numbers of P1A tetramer+ CD8+ T cells and the ratios of P1A tetramer+CD8+ T-cell number/weight (for P1A tetramer+ T-cell total number, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0110, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0078, vehicle + IgG vs dasatinib + anti-OX40: **P = .0024; for P1A tetramer+ T-cell number/gram, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0265, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0507, vehicle + IgG vs dasatinib + anti-OX40: *P = .0229). (D) Total numbers of IFN-γ+ CD8+ T cells and the ratios of IFN-γ+ CD8+ T-cell number/tumor weight (for IFN-γ+ T-cell total number, vehicle + anti-OX40 vs dasatinib + anti-OX40: ***P < .0001, dasatinib + IgG vs dasatinib + anti-OX40: ***P < .0001, vehicle + IgG vs dasatinib + anti-OX40: ***P < .0001; for IFN-γ+ T-cell number/gram, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0195, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0505, vehicle + IgG vs dasatinib + anti-OX40: *P = .0220).
Influence of dasatinib and anti-OX40 treatment on the tumor-infiltrating lymphocytes. (A) Effect of dasatinib and anti-OX40 on levels of the P1A-specific T cells in the blood. (B) Total numbers of CD8+ T cells and the ratios of CD8+ T-cell number/tumor weight (for CD8+ T-cell total number, vehicle + IgG vs vehicle + anti-OX40: ***P = .0007, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0250, vehicle + IgG vs dasatinib + anti-OX40: *P = .0165; for CD8+ T-cell number/gram, vehicle + IgG vs vehicle + anti-OX40: ***P = .0002, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0234, vehicle + IgG vs dasatinib + anti-OX40: **P = .0076). (C) Total numbers of P1A tetramer+ CD8+ T cells and the ratios of P1A tetramer+CD8+ T-cell number/weight (for P1A tetramer+ T-cell total number, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0110, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0078, vehicle + IgG vs dasatinib + anti-OX40: **P = .0024; for P1A tetramer+ T-cell number/gram, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0265, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0507, vehicle + IgG vs dasatinib + anti-OX40: *P = .0229). (D) Total numbers of IFN-γ+ CD8+ T cells and the ratios of IFN-γ+ CD8+ T-cell number/tumor weight (for IFN-γ+ T-cell total number, vehicle + anti-OX40 vs dasatinib + anti-OX40: ***P < .0001, dasatinib + IgG vs dasatinib + anti-OX40: ***P < .0001, vehicle + IgG vs dasatinib + anti-OX40: ***P < .0001; for IFN-γ+ T-cell number/gram, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0195, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0505, vehicle + IgG vs dasatinib + anti-OX40: *P = .0220).
The combination of dasatinib and anti-OX40 leads to up-regulation of IFN-γ– and IFN-γ–induced chemokines in the tumor microenvironment
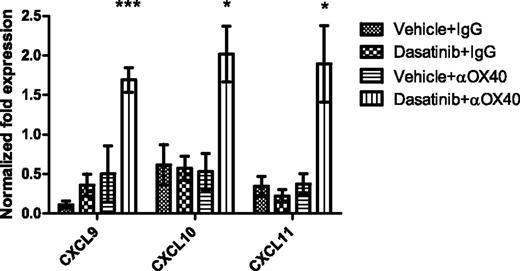
Because the repertoire of chemokines at the tumor site is an important determinant for whether sufficient CTL can be recruited to mediate a successful immune response, we next sought to determine whether the combination regimen impacted the chemokine profile within the tumor microenvironment. Quantitative RT-PCR was used to assess the transcript expression of a panel of chemokines. The results showed that the combination of dasatinib and anti-OX40 significantly increased the expression of the IFN-γ–induced chemokines CXCL9, 10, and 11 in the tumor microenvironment compared with other groups (Figure 6). The high levels of CXCL 9, 10, and 11 in the combination treatment group correlated with an increased effector: regulatory T-cell ratio, an increased proportion of tumor-infiltrating antigen-specific T cells, and improved therapeutic efficacy. These data suggest that induction of IFN-γ–associated chemokines may induce the migration of effector T cells into tumors to induce strong antitumor immunity following dasatinib plus anti-OX40 treatment.
Levels of CXCL9, 10, and 11 in tumors from 4 different groups. Tumors were harvested on day 16 after tumor inoculation. Real-time PCR was performed to detect the chemokine levels in the tumor microenvironment. GAPDH was used as the normalization reference gene. Graph shows the normalized fold expression of CXCL9, 10, and 11 (CXCL9: ***P = .0007, CXCL10: *P = .0327, CXCL11: *P = .0359).
Levels of CXCL9, 10, and 11 in tumors from 4 different groups. Tumors were harvested on day 16 after tumor inoculation. Real-time PCR was performed to detect the chemokine levels in the tumor microenvironment. GAPDH was used as the normalization reference gene. Graph shows the normalized fold expression of CXCL9, 10, and 11 (CXCL9: ***P = .0007, CXCL10: *P = .0327, CXCL11: *P = .0359).
Combining dasatinib with anti-OX40 leads to a “dual inhibition” of Treg function and increases the T-effector/Treg ratio in the tumor microenvironment
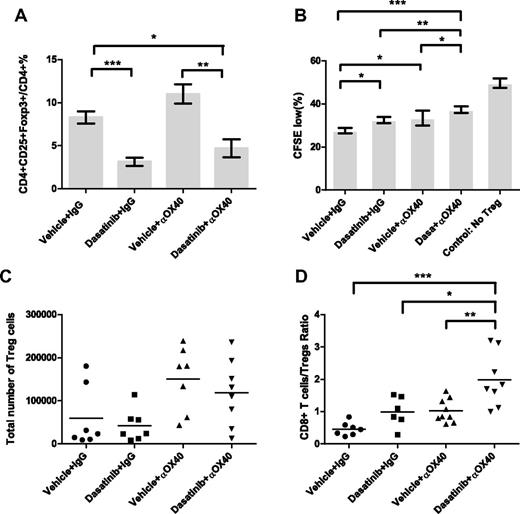
Next, we investigated the effect of this combination on Treg levels and function. Flow cytometric analysis of peripheral blood on day 11 showed that the addition of anti-OX40 to dasatinib did not further down-regulate Treg levels (Figure 7A), but Treg levels in the combination group remained much lower than that in the control group. Because both dasatinib and anti-OX40 have been shown to exert inhibitory effects on Treg function,41,42 we performed a Treg suppression assay to characterize Treg function in these mice. CD4+CD25high cells were isolated from the spleens of drug-treated mice and cultured with CFSE-labeled T effector cells, and the proliferation of the T effector cells was analyzed by flow cytometry 3 days later. We found that the combination of dasatinib and anti-OX40 resulted in Tregs with less suppressive function compared with the other 3 groups, suggesting that inhibition of Treg function provides an additional benefit of combining these 2 agents together. We also examined the effect of this combination on Treg levels in the tumor microenvironment on day 16. The numbers of the Treg cells infiltrating tumors were highly variable, with no statistically significant differences between the groups. However, we did find that the combination regimen significantly increased the ratio of CD8+ effector T cells to Treg cells within the tumor microenvironment (Figure 7D), which is known to be favorable for T cell–mediated tumor rejection.
Effect of dasatinib and anti-OX40 treatment on levels and functions of regulatory T cells. (A) Percentages of Treg cells in CD4+ T cells in the peripheral blood on day 11 (vehicle + IgG vs dasatinib + IgG: ***P = .0003, vehicle + anti-OX40 vs dasatinib + anti-OX40: **P = .0051, vehicle + IgG vs dasatinib + anti-OX40: *P = .0208). (B) Influence of dasatinib and anti-OX40 on functions of Tregs isolated from 4 different groups (vehicle + IgG vs dasatinib + IgG: *P = .0144, vehicle + IgG vs vehicle + anti-OX40: *P = .0254, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0019, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0488, vehicle + IgG vs dasatinib + anti-OX40: ***P = .0002). (C) Total numbers of Tregs in tumor infiltrating lymphocytes on day 16. (D) Ratios of CD8+ effector T cells/Treg cells in tumor-infiltrating lymphocytes on day 16 (vehicle + anti-OX40 vs dasatinib + anti-OX40: **P = .0065, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0228, vehicle + IgG vs dasatinib + anti-OX40: ***P = .0004).
Effect of dasatinib and anti-OX40 treatment on levels and functions of regulatory T cells. (A) Percentages of Treg cells in CD4+ T cells in the peripheral blood on day 11 (vehicle + IgG vs dasatinib + IgG: ***P = .0003, vehicle + anti-OX40 vs dasatinib + anti-OX40: **P = .0051, vehicle + IgG vs dasatinib + anti-OX40: *P = .0208). (B) Influence of dasatinib and anti-OX40 on functions of Tregs isolated from 4 different groups (vehicle + IgG vs dasatinib + IgG: *P = .0144, vehicle + IgG vs vehicle + anti-OX40: *P = .0254, dasatinib + IgG vs dasatinib + anti-OX40: **P = .0019, vehicle + anti-OX40 vs dasatinib + anti-OX40: *P = .0488, vehicle + IgG vs dasatinib + anti-OX40: ***P = .0002). (C) Total numbers of Tregs in tumor infiltrating lymphocytes on day 16. (D) Ratios of CD8+ effector T cells/Treg cells in tumor-infiltrating lymphocytes on day 16 (vehicle + anti-OX40 vs dasatinib + anti-OX40: **P = .0065, dasatinib + IgG vs dasatinib + anti-OX40: *P = .0228, vehicle + IgG vs dasatinib + anti-OX40: ***P = .0004).
Discussion
The therapeutic effects of targeted therapy are believed to be primarily dependent on direct proapoptotic and antiproliferative effects on tumor cells. However, our data suggest that tumor regression also strongly depends on the development of an antitumor immune response. In this study, we show that the antitumor effect of dasatinib in this model is likely to consist of direct tumoricidal effects combined with immunomodulatory effects of boosting antitumor T-cell responses, the latter of which is required for the maximal antitumor effects against P815 mastocytoma. These results highlight the importance of understanding how a targeted therapy drug effects the underlying immune response, and suggest that combinations of targeted drug and immune adjuvants may work well in synergy to achieve maximal antitumor efficacy.
Cytotoxic therapies cause the release of a plethora of proteins from tumor cells which may provide “foreign” antigens and thereby stimulate potent antitumor immunity. This has been demonstrated experimentally in several tumor models: small-molecule targeted therapy drugs, chemotherapy, or radiofrequency ablation generate tumor debris that can be taken up by the immune system to induce antitumor immune responses.43-47 Similarly, the increased tumor antigen-specific T-cell response we observed in dasatinib-treated mice might be partially caused by tumor antigens released from drug-induced apoptosis of tumor cells.
Early generations of targeted anticancer agents showed limited clinical efficacy that may have been partially because of off-target and deleterious effects on the immune system. Ideal targeted therapeutics that could be optimally combined with immunotherapy agents would include those that could block key oncogenic signaling pathways specifically in cancer cells, while preserving the function of immune cells. The immunomodulatory effects of dasatinib in vivo have yet to be fully characterized. It has been reported that dasatinib can inhibit the antigen-dependent expansion of T cells both in vitro and in vivo because of its inhibitory effect on the activity of Lck, a Src family member playing a critical role in TCR signaling, leading to the decreased activities of ERK and AKT and decreased cellular proliferation.34,35 However, clinical experience with patients show that dasatinib is more likely to provide immunomodulatory effects rather than causing severe immunosuppression. In a clinical study of dasatinib therapy for CML and Ph+ALL, a marked lymphocytosis caused by clonal expansion of T cells and NK cells was observed in patients treated with dasatinib.48 The discrepancy might lie in the differences in dose and duration of dasatinib treatment used in those studies. We found that short-term dasatinib treatment elicited a strong tumor antigen-specific CTL response, but paradoxically long-term dasatinib treatment inhibited the development of T-cell responses. Our studies suggest that a high-dose pulse of dasatinib treatment might be better than continuous treatment for preserving patients' immune systems and for combining dasatinib with immunotherapy. One study showing that cytotoxicity with transient potent target inhibition achieved by high-dose pulse dasatinib treatment is equivalent to prolonged target inhibition achieved by low-dose continuous dasatinib treatment in BCR-ABL–positive CML cell lines also suggests the potential clinical utility of intermittent high-dose-pulse dasatinib therapy.49 Therefore, we suggest that dasatinib can be used to improve the antitumor immune response when its duration and dose are optimized.
We also detected an overall decrease in the number of Treg cells following dasatinib treatment. Dasatinib has been shown to suppress the proliferation of Treg cells and inhibit Treg function in vitro.36 Our in vivo studies showed that Treg cells were much more sensitive to dasatinib inhibition than effector T cells and 3 days of dasatinib treatment could potentiate antigen-driven T-cell expansion in a tumor-free DC vaccine model. Therefore, suppression of Tregs might be another mechanism contributing to the enhanced tumor antigen-specific T-cell response induced by dasatinib treatment. Anti-OX40 has also been reported to inhibit Treg function.41,42 Consistent with this, our study showed that combination of dasatinib and anti-OX40 resulted in a “dual inhibition” of Treg function. Because effective antitumor immune responses are often blunted by immunosuppressive factors, an additional value of this combination may lie in its ability to suppress regulatory T cells.41,42
Migration of T cells into the tumor is essential for an effective antitumor response. Anti-OX40 has recently been shown to enhance CD8+ T-cell infiltration and decrease Treg infiltration into tumors in various tumor models.39,50 In our study, we found that anti-OX40 alone increased the percentage of CD8+ T cells and absolute number of CD8+ T cells per gram tumor. In the combination-treated mice, the CD8+ T-cell:Treg ratio was markedly increased and tumor antigen-specific T cells were enriched at the tumor site. Therefore, the function of anti-OX40 in this combination regimen seems to be potentiating the antitumor immune response by increasing the infiltration of CTL into the tumor microenvironment.
The repertoire of chemokines at the tumor site is an important factor influencing the migration and infiltration of T cells. The induction of CXCL9, 10, and 11 we observed with the combination regimen would be predicted to make the tumor microenvironment more permissive to infiltration by effector T cells. CXCL9, 10, and 11 are all IFN-γ–induced chemokines and they all use the receptor CXCR3 which is preferentially expressed by Th1 cells. In our experiments, the increased levels of Th1 chemokines in the combination treatment group is consistent with the high levels of CTL infiltration and supports the notion that treatment-mediated induction of Th1 chemokines favors the antitumor immune response. It has been reported that OX40 agonist therapy can enhance the expression of CXCL9 in the tumor microenvironment, suggesting that this therapy promotes effector T-cell trafficking by augmenting chemokine signaling at the tumor site.50 Therefore, our data are consistent with a model whereby dasatinib treatment leads to tumor antigen release from dying tumor cells and significant down-regulation of immune-suppressive components that in turn help with T-cell priming, leading to an increase of circulating tumor antigen-specific T cells. Anti-OX40, as a Treg inhibitor, works together with dasatinib to exert “dual inhibition” on Treg function, and also up-regulates chemokines at the tumor site to mediate the infiltration of antigen-specific T cells. This leads to increased levels of IFN-γ in the tumor microenvironment and further up-regulation of CXCL 9, 10, and 11, thereby creating a positive feedback loop that attracts more effector T cells into the tumor microenvironment. This combination works on both priming and trafficking stages of the antitumor T-cell response and provides a very promising therapeutic effect.
In summary, we have shown that the antitumor effects of dasatinib are largely dependent on CD8+ T cell–mediated immunity, and that enhancing this T cell response with anti-OX40 can greatly increase the therapeutic effect. Combining a targeted tumoricidal agent with an immune-potentiating drug represents an important emerging strategy for cancer treatment. Although our data suggest that coadministration of targeted therapy drugs and immune-stimulating agents requires optimization of dosing and schedule to achieve maximal antitumor efficacy, overall the evidence supports moving this combination into human clinical trials for cancer patients.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by National Institutes of Health/National Cancer Institute grants P50 CA093459, P01CA128913, R01CA143077, and R01CA58033.
National Institutes of Health
Authorship
Contribution: Y.Y. designed and performed experiments, analyzed data, and wrote the manuscript; C.L. designed and performed experiments; W.P. designed the real-time PCR primer, provided reagents, and designed experiments; G.L. provided critical suggestions and wrote the manuscript; W.W.O. provided critical suggestions; Y.L. provided P1CTL transgenic mice and provided critical suggestions; S.E.W. provided critical suggestions and wrote the manuscript; and P.H. designed experiments, reviewed data, and wrote the manuscript.
Conflict-of-interest disclosure: S.E.W. has a Bristol-Myers Squibb research grant. The remaining authors declare no competing financial interests.
Correspondence: Patrick Hwu, Professor, Chair, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: phwu@mdanderson.org.