Abstract
Hematopoietic stem cells (HSCs) constitute a rare population of tissue-specific cells that can self-renew and differentiate into all lineages of the blood cell system. These properties are critical for tissue regeneration and clinical applications of HSCs. Cord blood is an easily accessible source of HSCs. However, the number of HSCs from one unit is too low to effectively transplant most adult patients, and expansion of HSCs in vitro has met with limited success because of incomplete knowledge regarding mechanisms regulating self-renewal. Members of the TGF-β superfamily have been shown to regulate HSCs through the Smad signaling pathway; however, its role in human HSCs has remained relatively uncharted in vivo. Therefore, we asked whether enforced expression of the common-Smad, Smad4, could reveal a role for TGF-β in human hematopoietic stem/progenitor cells (HSPCs) from cord blood. Using a lentiviral overexpression approach, we demonstrate that Smad4 overexpression sensitizes HSPCs to TGF-β, resulting in growth arrest and apoptosis in vitro. This phenotype translates in vivo into reduced HSPC reconstitution capacity yet intact lineage distribution. This suggests that the Smad pathway regulates self-renewal independently of differentiation. These findings demonstrate that the Smad signaling circuitry negatively regulates the regeneration capacity of human HSPCs in vivo.
Introduction
Hematopoiesis, the process of blood-cell formation, relies on a rare population of multipotent hematopoietic stem cells (HSCs) that reside in the BM of adult persons. HSCs are somatic stem cells that carry the dual capacity to self-renew, thus maintaining the stem cell pool over time, and to differentiate into all mature lineages of the hematopoietic system. These properties are absolutely essential for hematopoietic regeneration and subsequent therapeutic applications of HSCs in the clinic. Self-renewal and differentiation are under strict control by both cell-intrinsic and external factors in the BM niche.1,2 Cord blood (CB) constitutes an abundant source of HSCs that can be used in allogeneic BM transplants.3 However, the yield of HSCs from CB is too low to efficiently transplant a majority of adult patients. As efforts to expand HSCs in vitro have been hampered by limited knowledge regarding the regulation of proliferation and self-renewal, CB remains a source of HSCs not fully exploited for clinical purposes.
Members of the TGF-β superfamily, including TGF-β, activins, and bone morphogenetic proteins (BMPs), regulate a wide array of cellular processes, such as apoptosis, proliferation, and differentiation.4-7 The TGF-β superfamily has been shown to have distinct and highly context dependent effects on HSCs. For example, BMP4 has been implicated as a positive regulator of proliferation and survival of human CB hematopoietic stem/progenitor cells (HSPCs),8 whereas TGF-β1 is a well-established negative regulator of HSPC growth, affecting cell cycle and apoptotic parameters.9-12
TGF-β ligands convey signals intracellularly through the Smad signaling pathway. The Smad proteins can be divided into 3 functional classes depending on their role in the pathway: the receptor activated Smads (R-Smad; Smad1, 2, 3, 5, and 8), the common-mediator Smad (Co-Smad; Smad4), and the inhibitory Smads (I-Smad; Smad6 and 7). Ligand binding followed by receptor phosphorylation activates the intracellular R-Smads, which then form a complex with Smad4. The activated complex subsequently translocates into the nucleus where it regulates transcription of target genes. R-Smad2 and 3 operate downstream of TGF-β and activin receptors, whereas R-Smad1, 5, and 8 primarily act downstream of BMP receptors. The I-Smads function to inhibit Smad signaling by interacting with the Co-Smad or competing with the R-Smad receptor binding. Depending on the combination of ligands and receptors that interact, different Smad proteins are activated specifying the signaling outcome.6 Recently, the entire Smad signaling pathway was inhibited through overexpression of the inhibitory Smad7 in murine HSCs by retroviral gene transfer. This resulted in increased self-renewal of HSCs in vivo with no abnormalities in differentiation of the myeloid and lymphoid lineages.13 However, Smad7 overexpression did not result in increased self-renewal of HSCs in vitro, indicating that the in vivo phenotype was dependent on the environment of the BM niche. Moreover, an MxCre inducible knockout model targeting Smad4, thus disrupting the entire Smad pathway, led to a loss of self-renewal in murine HSCs in vivo, whereas in vitro studies showed that the proliferation capacity was normal.14 Importantly, Smad7 overexpression in Smad4-deficient HSCs demonstrated that the Smad7-induced regeneration capacity was dependent on Smad4, suggesting that the level at which the Smad pathway is disrupted is important for the effect on HSCs.13 These findings demonstrate the complexity of Smad signaling and highlight the importance to investigate it further.
Although TGF-β has been shown in several studies to inhibit human HSPC growth in vitro, notably very little is known regarding similar effects in vivo.9,10,15-20 Therefore, we asked whether enforced expression of Smad4 could reveal a role for TGF-β in human HSPC regulation in vivo, uncovering a role in self-renewal and regenerative ability of HSPCs after transplantation. We report here that increased Smad4 expression sensitizes human CB HSPCs to TGF-β. This leads to growth arrest and apoptosis in vitro and reduced HSPC reconstitution capacity in vivo with no effect on lineage distribution. Our study demonstrates that the level of Smad4 determines the strength of the TGF-β signal and indicates that Smad4 is a limiting factor in the Smad signaling pathway. Furthermore, the present study reveals an important role for the Smad signaling circuitry as a negative regulator of human HSPCs in vivo during regeneration of the hematopoietic system.
Methods
CB
CB was obtained from Lund University Hospital, Sweden, in accordance with procedures approved by the human ethics committee. For all experiments, CB samples were used within 24 hours of delivery.
Purification of CD34+ cells from CB
Mononuclear cells were purified from CB using Lymphoprep (Axis-Shield) and density gradient centrifugation according to the manufacturer's instruction. Cells were then pooled in PBS (Invitrogen) with 2mM EDTA (Invitrogen) and 5% FCS (Invitrogen), and washed once. The pellet was resuspended in 300 μL PBS/EDTA/FCS and mixed with 100 μL FcR Blocking Reagent (QIAGEN). Cells were incubated with 100 μL CD34-conjugated MicroBeads (QIAGEN) on shaker at 4°C for 30 minutes. After washing, a MidiMACS column (QIAGEN) was used to purify CD34+ cells, according to the manufacturer's instructions.
Transduction
A lentiviral vector containing the green fluorescent protein (GFP) with or without the 3.2-kb human Smad4 full-length cDNA fragment was used. The original vector was a kind gift from Christopher Baum, Hannover Medical School. Before transduction, human CB cells were prestimulated for 24 hours in serum-free expansion medium (SFEM; StemCell Technologies) containing 100 IU penicillin, 100 μg streptomycin (P/S; Invitrogen), supplemented with human stem cell factor (hSCF, 100 ng/mL, PreproTech), human Thrombopoietin (hTPO, 100 ng/mL, PreproTech), human Flt3 ligand (Flt3L, 100 ng/mL, PreproTech). Before transduction, a 48-well nontissue culture plate (BD Biosciences) was incubated at room temperature with retronectin (Takara Bio) for 2 hours followed by a 30-minute block with 2% BSA (Stem Cell Technologies). Subsequently, 1 × 105- × 106 CB cells were transduced for 24 hours (MOI: 5-50). Transduction was carried out in SFEM with P/S (Invitrogen), hSCF (100 ng/mL, PreproTech), hTPO (100 ng/mL, PreproTech) and Flt3L (100 ng/mL, PreproTech). GFP expression was measured by flow cytometry 2-3 days after transduction to estimate transduction efficiencies.
Mice
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were bred in the animal facility at the biomedical center, Lund University. Mice were kept in ventilated racks and given autoclaved food and water. Lund University's ethical committee approved all animal experiments.
Transplantations
One day after lentiviral transduction, 1-2 × 105 unsorted CB cells were transplanted into sublethally irradiated (300 cGy) NSG mice. Recipient mice were killed 6 months after transplantation and femur and tibiae were crushed in a mortar to collect BM cells. Cell suspensions were filtered through a 70-μm nylon cell strainer (BD Biosciences) in PBS (Invitrogen) containing 2% FCS (Invitrogen) and analyzed by flow cytometry.
Cell preparations
Peripheral blood (PB) was collected from the tail veins and kept in heparin (2000 IU/mL; Leo Pharma) until further analysis. When necessary, red blood cells were lysed with ammonium chloride (NH4Cl; StemCell Technologies). BM was harvested as described in “Mice.”
Flow cytometry
For FACS analysis, fluorochrome-conjugated monoclonal antibodies against human cell surface markers were used; allophyocyanin-conjugated hCD34, hCD45 (BD Biosciences), PE-conjugated hCD90, hCD19, hCD15, hCD13 (BD Biosciences), V450-conjugated hCD45RA (BD Biosciences), and FITC-conjugated hCD38 (BD Biosciences). Dead cells were detected by staining with 7-aminoactinomycin D (7-AAD; Sigma-Aldrich). Cells were sorted on a FACSVantage Cell Sorter (BD Biosciences) and analyzed using FACSCanto (BD Biosciences). Resulting data were reanalyzed using FlowJo Version 9.4 software (TreeStar).
In vitro culture and colony assays
After transduction, cells were cultured in SFEM (StemCell Technologies) with P/S (Invitrogen), supplemented with hSCF (100 ng/mL, PreproTech), hTPO (100 ng/mL, PreproTech), and Flt3L (100 ng/mL, PreproTech). CB cells were incubated at 37°C in 5% CO2. Cells were passaged and analyzed by FACS at indicated time points. As a quality control of the CB preparations, units where both control- and Smad4-transduced cells did not increase in number between day 3 and 6 of culture, were excluded from the experiment. For growth inhibition assays, 0.05, 0.1, or 5 ng/mL human TGF-β1 (R&D Systems) was added to the media, with or without 1 or 5μM TGF-β receptor inhibitor targeting ALK4, 5, and 7 receptors (StemoleculeSB431542; Stemgent). For colony assays, 500-750 GFP-sorted cells were plated in 35-mm Petri dishes in methylcellulose medium (M4230; StemCell Technologies) containing hSCF (25 ng/mL), GM-CSF (50 ng/mL, R&D), hIL-3 (25 ng/mL, PreproTech), hEPO (5 U/mL, Apoteket Farmaci), and P/S (Invitrogen). For colony assays with BMP, 50 ng/mL of BMP-4/7 heterodimer (R&D Systems) was used. Total colony number was scored after 14 days of culture.
Western blot
Transduced CB cells were grown for 2-3 days after transduction followed by flow cytometry sorting to purify GFP+ cells. Cells were collected and lysed in Leammli buffer (Bio-Rad) supplemented 1.5% β-mercaptoethanol (Sigma-Aldrich). Samples were then boiled and loaded on an acrylamide gel (Invitrogen). The proteins were transferred to a nitrocellulose membrane (Invitrogen) and incubated with mouse anti-Smad4 (Santa Cruz Biotechnology) in 5% dry milk overnight. As a secondary antibody, antimouse IgG horseradish peroxidase (GE Healthcare) was used. For loading control, the membrane was washed with Restore Western blot Stripping Buffer (Thermo Scientific) and incubated with mouse antiactin (Santa Cruz Biotechnology) followed by secondary antibody.
Quantitative RT-PCR
RNA from sorted GFP+ CB cells was isolated (RNeasy; QIAGEN) and reverse transcribed (SuperScript III, Invitrogen) in the presence of random hexamers. Quantitative PCRs (TaqMan; Applied Biosystems) were performed in an ABI Prism 7700 (Applied Biosystems) according to the manufacturer's protocol with gene-specific primers (Applied Biosystems). Each assay was performed in triplicate, and the results were normalized to the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (Hprt). For quantitative RT-PCR using microfluidic technique, CD34+CD38−CD90+CD45RA− CB cells were sorted and transduced as described in “Transduction.” Three days after transduction, 50 GFP+ cells were directly sorted into 4 μL lysis buffer, containing 0.4% NP40, deoxynucleoside triphosphates, dithiothreitol, and RNase OUT (Invitrogen), and snap frozen in −80°C. On thawing, 8.25 μL of CellsDirect reaction mix containing 1 μL of SSIII/PlatinumTaq (CellsDirect One-Step RT_qPCR kit, no ROX, Invitrogen) and 46 TaqMan assays (Applied Biosystems; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) to a final dilution of 0.05× each were added to the cell lysate for RT-PCR before amplification and gene expression quantification according to previously published protocols.21
Cell cycle analysis
Transduced CB cells were cultured for 6 days and then analyzed with respect to cell cycle status (n = 4). For in vivo experiments, CD34+GFP+ cells from BM were analyzed 5 weeks after transplantation (n = 3). Cells were fixed using 0.4% paraformaldehyde (Sigma-Aldrich) in PBS (Invitrogen) for 30 minutes at room temperature, and then incubated with an equal volume of 0.2% Triton-X (Sigma-Aldrich) for one hour at 4°C. The fixed and permeabilized CB cells were washed, resuspended in PBS (Invitrogen), and stained with Ki67-PE (BD Biosciences) for 2 hours. Cells were washed and resuspended in PBS (Invitrogen) containing 7-AAD (Sigma-Aldrich) for one hour at 4°C. Gated GFP+ cells were analyzed for Ki67 expression and 7-AAD incorporation using FACSCanto (BD Biosciences).
Apoptosis assay
For apoptosis assays, transduced CB cells cultured for 6 days were analyzed (n = 5). In vivo, CD34+GFP+ cells from BM were analyzed 5 weeks after transplantation (n = 3). Cells were washed twice and resuspended in staining buffer containing annexin V–PE and 7-AAD (BD Biosciences) according to the manufacturer′s protocol (BD Biosciences). Cells were incubated for 15 minutes, and subsequently GFP+ cells were analyzed for annexin V binding and 7-AAD incorporation.
Statistical analysis
Data were analyzed using paired Student t test or Mann-Whitney test. P ≤ .05 was considered significant.
Results
Smad4 is efficiently expressed in human CD34+ CB cells using a lentiviral vector
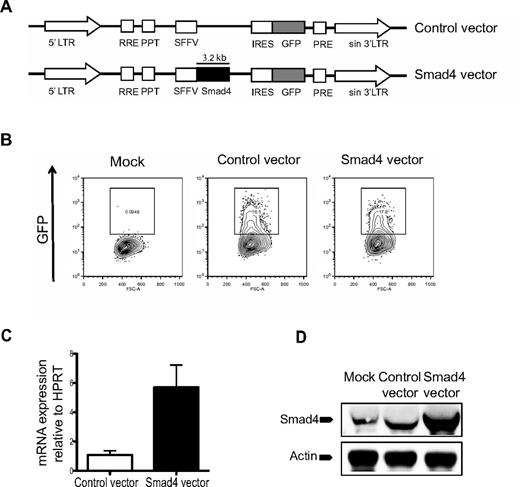
To investigate the effect of Smad4 overexpression on hematopoiesis, CD34+ CB-derived hematopoietic cells were transduced with lentiviral Smad4 or control vectors (Figure 1A). Transduction efficiencies were similar between control and Smad4 vector-transduced cells, as measured by flow cytometric analysis of GFP expression (Figure 1B). To confirm Smad4 overexpression at the mRNA level, transduced GFP+ cells were sorted and subjected to RT-PCR analysis, which demonstrated a > 5-fold increase in expression of Smad4 compared with control-transduced cells (Figure 1C). Furthermore, Western blot analysis of GFP+ transduced CD34+ CB cells revealed robust overexpression of Smad4 at the protein level (Figure 1D). Taken together, these results confirm that the overexpression construct used in this study generates increased expression of Smad4 both at mRNA and protein levels.
Vector design and evidence for Smad4 overexpression in human cells. (A) Schematic design of control and Smad4 vector constructs. LTR indicates long terminal repeats. (B) Transduction efficiencies of CB cells were determined 2-3 days after transduction. (C) RT-PCR results of Smad4 mRNA expression in CB HSCs. Values are relative to HPRT expression. (D) Western blot analysis showing Smad4 protein expression in human CB cells. Actin was used as loading control.
Vector design and evidence for Smad4 overexpression in human cells. (A) Schematic design of control and Smad4 vector constructs. LTR indicates long terminal repeats. (B) Transduction efficiencies of CB cells were determined 2-3 days after transduction. (C) RT-PCR results of Smad4 mRNA expression in CB HSCs. Values are relative to HPRT expression. (D) Western blot analysis showing Smad4 protein expression in human CB cells. Actin was used as loading control.
High levels of Smad4 result in increased sensitivity to TGF-β
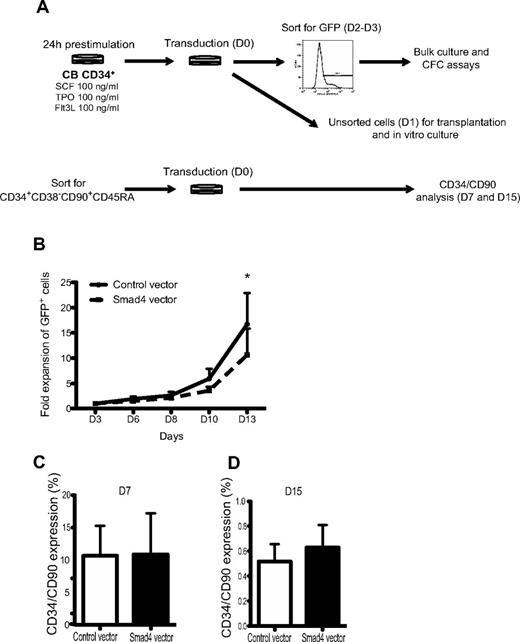
To determine the effect of Smad4 overexpression on human hematopoietic progenitor growth, transduced CD34+ CB-derived cells were assayed with respect to proliferative and clonogenic properties in vitro (Figure 2A). Over the course of 13 days, the proliferative capacity of Smad4-transduced cells was significantly decreased compared with control-transduced cultures (Figure 2B). To determine whether or not enforced expression of Smad4 would affect the proportion of primitive cells in vitro, CD34+CD38−CD90+CD45RA− CB cells were sorted before transduction (Figure 2A). After 7 and 15 days of in vitro culture, HSPCs were analyzed with respect to CD34 and CD90 cell surface expression. No significant differences in proportion of primitive cells were observed between control and Smad4 vector-transduced cells (Figure 2C-D). However, as enforced expression of Smad4 resulted in a reduced proliferative capacity, the total number of CD34+CD90+ cells were reduced compared with control-transduced cultures (data not shown). Together, these data indicate that enforced expression of Smad4 in human CB HSPC impair their proliferative capacity but does not change the proportion of primitive cell surface markers during in vitro culture.
Decreased growth of GFP+ human CB cells in vitro. (A) Experimental outline. (B) Unsorted CD34+ CB cells were plated in serum-free media. At given time points, GFP percentages were analyzed by FACS, and cells were counted and passaged. Data represent fold increase in number of cells ± SEM (n = 3). *P = .05 (paired Student t test). (C) FACS analysis showing CD34/CD90 expression after 7 and 15 days (D) of culture.
Decreased growth of GFP+ human CB cells in vitro. (A) Experimental outline. (B) Unsorted CD34+ CB cells were plated in serum-free media. At given time points, GFP percentages were analyzed by FACS, and cells were counted and passaged. Data represent fold increase in number of cells ± SEM (n = 3). *P = .05 (paired Student t test). (C) FACS analysis showing CD34/CD90 expression after 7 and 15 days (D) of culture.
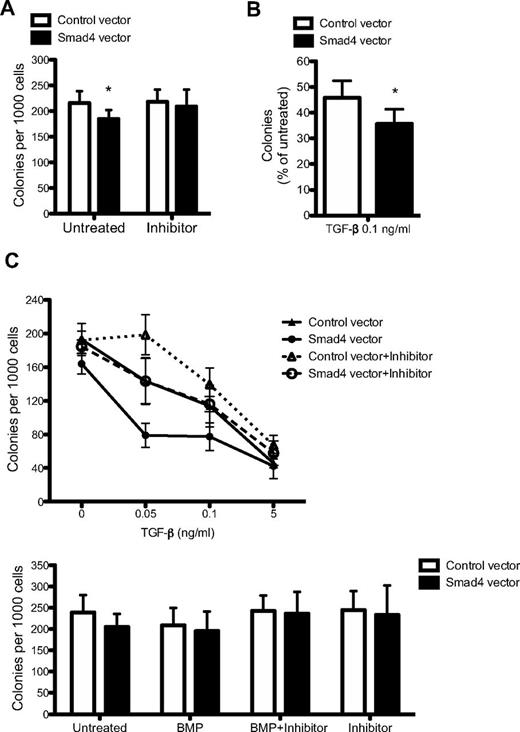
Given the fact that Smad4 is an integral component of the signaling cascade downstream of TGF-β, we sought to evaluate whether or not overexpression of Smad4 resulted in an altered response to TGF-β–mediated growth inhibition. Therefore, the sensitivity to TGF-β was investigated in methylcellulose cultures. To study changes in colony-forming capacity of GFP-sorted cells, methylcellulose cultures supporting both myeloid and erythroid colonies were seeded 2-3 days after transduction. Enforced expression of Smad4 resulted in a significant decrease in colony frequency (Figure 3A). Importantly, this phenotype could be rescued on addition of the TGF-β type I receptor inhibitor, SB431542 (Figure 3A; supplemental Figure 1). Furthermore, Smad4-transduced cells showed an increased sensitivity to TGF-β, resulting in a higher degree of inhibition compared with control-transduced cells at a TGF-β concentration of 0.1 ng/mL (Figure 3B). To fully evaluate the TGF-β sensitivity of Smad4-overexpressing cells, a dose-response experiment to TGF-β with or without the SB431542 inhibitor was performed. At low concentrations of TGF-β, the hypersensitivity of Smad4-overexpressing cells was still distinct and could be abolished by addition of the SB431542 inhibitor, thus verifying the functionality and specificity of the inhibitor (Figure 3C). At higher concentrations of TGF-β, cells become saturated, such that the difference between control and Smad4-overexpressing cells flattened out (Figure 3C).
Smad4 overexpression in CB cells results in increased sensitivity to TGF-β. (A) CD34+ CB cells were sorted for GFP 2-3 days after transduction and plated in methylcellulose supplemented as indicated with inhibitor SB431542. Data represent number of colonies per 1000 cells plated ± SEM (n = 6). (B) Percentage of colonies formed after 0.1 ng/mL TGF-β treatment compared with untreated cultures (n = 6). (C) Dose-response curve to TGF-β plus (dotted lines) or minus (solid lines) inhibitor SB431542 (n = 3). (C) GFP+ cells were plated in methylcellulose supplemented with BMP-4/7 heterodimer alone or together with inhibitor SB431542 (n = 3). *P ≤ .05 (paired Student t test).
Smad4 overexpression in CB cells results in increased sensitivity to TGF-β. (A) CD34+ CB cells were sorted for GFP 2-3 days after transduction and plated in methylcellulose supplemented as indicated with inhibitor SB431542. Data represent number of colonies per 1000 cells plated ± SEM (n = 6). (B) Percentage of colonies formed after 0.1 ng/mL TGF-β treatment compared with untreated cultures (n = 6). (C) Dose-response curve to TGF-β plus (dotted lines) or minus (solid lines) inhibitor SB431542 (n = 3). (C) GFP+ cells were plated in methylcellulose supplemented with BMP-4/7 heterodimer alone or together with inhibitor SB431542 (n = 3). *P ≤ .05 (paired Student t test).
As Smad4 functions as a key component in the BMP signaling pathway, we further tested whether the colony-forming capacity in response to BMP was altered on overexpression of Smad4. However, no differences could be observed in colony formation on addition of BMP-4/7 heterodimer, either with or without the inhibitor SB431542, compared with control-transduced cells (Figure 3D). Thus, overexpression of Smad4 in human CD34+ CB cells resulted in increased sensitivity to TGF-β, which could be rescued by addition of the TGF-β type I receptor inhibitor, SB431542. These results establish that the exact concentration of Smad4 is critically important for the responsiveness to TGF-β in human cells from CB and demonstrate that hematopoietic progenitors become hypersensitive to TGF-β on increasing levels of Smad4.
Overexpression of Smad4 results in altered cell cycle and apoptotic properties in a TGF-β–dependent fashion
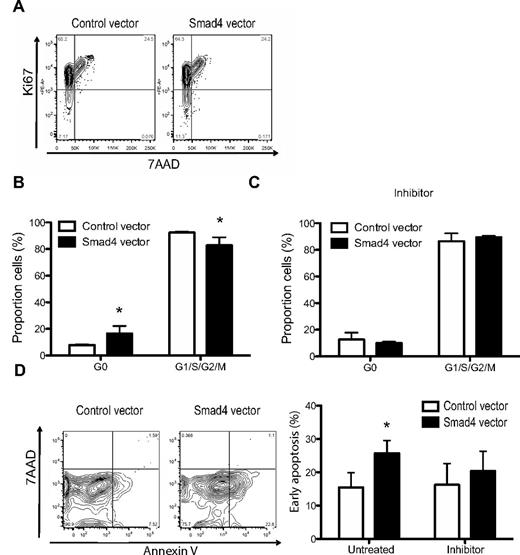
It has previously been shown that TGF-β inhibits HSPC growth through effects on cell cycle.9,10 To gauge the impact of Smad4 overexpression on the cell cycle of CD34+ CB-derived cells, transduced cells were analyzed in vitro (Figure 4A). Cells with enforced expression of Smad4 exhibited a significant 2-fold increase in the proportion of cells in a quiescent state of the cell cycle (G0) compared with control cells when cultured for 6 days in vitro (Figure 4B). Importantly, Smad4-overexpressing cells could be released from G0 on addition of the TGF-β type I receptor inhibitor, SB431542 (Figure 4C), suggesting that even small amounts of autocrine TGF-β are enough to alter the cell cycle status. Moreover, as TGF-β is known to inhibit growth through apoptosis,11 we further investigated whether enforced expression of Smad4 affected apoptosis in cultured CB cells. After 6 days of culture, Smad4-overexpressing cells had significantly higher annexin V binding compared with control cells (Figure 4D-E), an effect that was reversed on addition of the inhibitor SB431542 to the culture. These results demonstrate that enforced expression of Smad4 leads to increased sensitivity to TGF-β, translating into functional changes in cell cycle and apoptotic parameters in vitro.
Shifted cell cycle status and increased apoptosis in TGFβ-hypersensitive cells. (A) FACS analysis of unsorted transduced CD34+ CB cells cultured under serum-free conditions for 5-6 days. (B) Average percentages of cell cycle status in GFP+ cells without or (C) with inhibitor SB431542, ± SEM (n = 4). (D) Representative FACS plots and (E) average percentages of GFP+ cells in early apoptosis (annexin V+7–AAD−) with or without inhibitor SB431542, ± SEM (n = 5). *P ≤ .05 (paired Student t test or the Mann-Whitney test).
Shifted cell cycle status and increased apoptosis in TGFβ-hypersensitive cells. (A) FACS analysis of unsorted transduced CD34+ CB cells cultured under serum-free conditions for 5-6 days. (B) Average percentages of cell cycle status in GFP+ cells without or (C) with inhibitor SB431542, ± SEM (n = 4). (D) Representative FACS plots and (E) average percentages of GFP+ cells in early apoptosis (annexin V+7–AAD−) with or without inhibitor SB431542, ± SEM (n = 5). *P ≤ .05 (paired Student t test or the Mann-Whitney test).
Human CD34+ CB cells overexpressing Smad4 exhibit impaired repopulative capacity on xenograft transplantation
Previous reports have shown that TGF-β regulates human HSPCs in vitro, but information is still lacking concerning the role of TGF-β in vivo.9,10,15-20,22 Having established that CB-derived HSPCs become hypersensitive to TGF-β in vitro on overexpression of Smad4, we sought to use this approach to evaluate how TGF-β functions in a transplantation setting in vivo. Therefore, we transplanted unsorted transduced CD34+ CB cells into 3 or 4 sublethally irradiated NSG-mice in each of 4 independent experiments. The total engraftment of human CD45+ cells was similar in both control and Smad4-overexpressing cells (Figure 5A). However, despite having similar transduction efficiencies (27% ± 8.35% vs 27% ± 7.32% for Smad4 vector and control vector, respectively), CB HSPCs transduced with the Smad4 vector exhibited a significantly impaired engraftment capacity as measured by FACS analysis of PB at 7, 12, and 25 weeks after transplantation (Figure 5C; 7 weeks, 3-fold; 12 weeks, 4-fold; and 25 weeks, 3-fold). Our data indicate that it is unlikely that Smad4 overexpression had an effect on short-term stem cells and progenitors as engraftment 3 weeks after transplantation was similar in both control and Smad4-overexpressing cells. In alignment with data from PB, long-term analysis in BM 25 weeks after transplantation showed that engraftment of Smad4-overexpressing cells was significantly decreased compared with control-transduced cells (Figure 5D). In addition, to investigate whether TGF-β hypersensitivity in human HSPCs would change the differentiation potential of cells in vivo, we analyzed the proportion of lymphoid and myeloid cells in PB by FACS. Importantly, Smad4-overexpressing cells gave rise to both CD13+/CD15+ myeloid cells and CD19+ B-cells at similar proportions as control-transduced cells (Figure 5F). Likewise, the proportion of CD34+ cells in BM 25 weeks after transplantation was comparable between Smad4 and control-transduced cells. However, because the percentage of GFP was significantly lower in the BM of persons transplanted with Smad4 vector-transduced cells 25 weeks after transplantation, not enough data points were generated to provide a robust analysis on the proportion of CD34 expression (Figure 5E). Instead, to determine whether overexpression of Smad4 would impact the proliferation of HSPCs in vivo during the regeneration process, we performed cell cycle and apoptosis analyses on CD34+GFP+ cells in BM 5 weeks after transplantation. Similar to what was observed in vitro, apoptosis was increased and more cells resided in G0 on overexpression of Smad4 in vivo (supplemental Figure 2). Together, these data demonstrate that increased sensitivity to TGF-β inhibits HSPC reconstitution capacity in vivo but has no effect on lineage distribution. To get molecular insights into the mechanisms regulating these effects, we isolated mRNA from control vector–transduced or Smad4 vector–transduced putative HSCs and quantitatively analyzed expression levels of a range of genes based on their documented role in apoptosis, cell cycle, or TGF-β signaling using the Fluidigm dynamic array platform (supplemental Table 1; supplemental Figure 3). Interestingly, the ETS family transcription factor Pu.1, previously shown to be indispensible for murine HSC reconstitution capacity and ability to generate the earliest myeloid and lymphoid progenitors,23 was significantly > 2-fold down-regulated in Smad4-overexpressing cells (P < .05), possibly revealing an important downstream molecule for TGF-β/Smad signaling in HSCs. In addition, although mRNA levels of the remaining genes were unchanged (data not shown) or not statistically significant on enforced Smad4 expression, a mild up-regulation of the cell cycle inhibitor p21, the TGF-β target Smad6, and the positive regulator of apoptosis growth arrest and DNA-damage-inducible β (Gadd45b) was detected (supplemental Figure 3). Because these genes have previously been identified as downstream targets of TGF-β signaling, they are likely to also play a part in the observed phenotypes.24-27
Smad4-overexpressing cells exhibit impaired repopulative capacity on xenograft transplantation. (A) CD34+ CB cells were transduced and unsorted cells were transplanted into NSG mice one day after transduction. The graph shows total human engraftment after 25 weeks in BM. (B) Representative FACS plots of GFP expression in recipient mice. (C) GFP+ cells present in PB after 3, 7, 12, and 25 weeks after transplantation. (D) Percentages of GFP+ cells in BM and (E) FACS analysis of CD34 expression in GFP+ cells from BM 25 weeks after transplantation. (F) PB lineage distribution of control and Smad4-transduced cells after 12 weeks in PB, ± SEM (n = 4). **P ≤ .01 (Mann-Whitney test). ***P ≤ .001 (Mann-Whitney test).
Smad4-overexpressing cells exhibit impaired repopulative capacity on xenograft transplantation. (A) CD34+ CB cells were transduced and unsorted cells were transplanted into NSG mice one day after transduction. The graph shows total human engraftment after 25 weeks in BM. (B) Representative FACS plots of GFP expression in recipient mice. (C) GFP+ cells present in PB after 3, 7, 12, and 25 weeks after transplantation. (D) Percentages of GFP+ cells in BM and (E) FACS analysis of CD34 expression in GFP+ cells from BM 25 weeks after transplantation. (F) PB lineage distribution of control and Smad4-transduced cells after 12 weeks in PB, ± SEM (n = 4). **P ≤ .01 (Mann-Whitney test). ***P ≤ .001 (Mann-Whitney test).
Conclusively, our results establish that Smad4 is a limiting factor in the TGF-β signaling pathway and that Smad signaling inhibits the repopulative capacity of human HSPCs in vivo after transplantation.
Discussion
A deeper understanding of the regulation of self-renewal and proliferation of human HSPCs in vivo is of great importance for the purpose of developing more efficient ex vivo expansion protocols of HSPCs in the future. TGF-β is considered one of the most potent growth inhibitory factors of both human and mouse HSCs in vitro. In mouse, overexpression of the inhibitory Smad7 has been demonstrated to increase self-renewal of HSCs in vivo, indicating that the Smad pathway regulates self-renewal negatively.13 However, a conditional knockout mouse model targeted to Smad4, thus entirely disrupting the Smad pathway, showed reduced repopulative capacity in the Smad4-deficient HSCs.14 These findings demonstrate the complexity of the Smad signaling pathway and highlight the importance of further investigation. Recently, it was also published that TGF-β signaling is active in murine LT-HSCs under neural control in vivo and that this signaling is critical for HSC maintenance.28 However, very little is known about the downstream Smad effectors in human cells. In an effort to decipher the role of the Smad signaling pathway in human HSPCs, we have studied the effect of enforced Smad4 expression, the key component of the Smad signaling circuitry, in human cells from CB. Interestingly, on overexpression of Smad4, HSPCs became hypersensitive to the growth inhibitory effects of TGF-β, translating in reduced reconstitution potential in vivo (Figure 6). This observation suggests that Smad4 is a limiting factor in the TGF-β signaling pathway and that the precise level of Smad4 can modulate the response to TGF-β in human cells. We attempted to use this as a model to evaluate how and if TGF-β functions in vivo in a setting where HSPCs are being transplanted. We hypothesized that, if TGF-β functions to affect fate options, such as self-renewal and/or differentiation capacity of HSPCs in vivo, overexpression of Smad4 could be used to shed light on such a mechanism. Indeed, after transplantation into NSG-mice Smad4-overexpressing cells exhibited a significantly reduced repopulation capacity already after 7 weeks after transplantation. This phenotype was sustained overtime, revealing a reduced engraftment capacity in PB at 12 and 25 weeks, and in BM at 25 weeks. Importantly, as engraftment 3 weeks after transplantation was similar for both control and Smad4-transduced cells, our results indicate that Smad4 overexpression affects long-term stem cells but keeps the short-term stem cells unaffected. Indeed, Yamazaki et al recently showed that active TGF-β is present close to dormant long-term stem cells.28 In addition, in the mouse system, it has recently been shown that high levels of TGF-β1 invariably result in inhibition of primitive hematopoietic cells. In contrast, low levels of TGF-β1 can be either stimulatory, as in the case of the most repopulation-competent myeloid-biased LT-HSCs, or inhibitory, as is the case for more mature progenitors.29 Our data presented here support the idea that the precise level of TGF-β/Smad4 signaling is critical for LT-HSC function. Previous results derived from Smad4 null HSCs showed no abnormalities in lineage distribution after transplantation, and neither did the study in which Smad7 was overexpressed.13,14 In contrast, another Smad7 overexpression study showed that human SCID-repopulating cells differentiated at a higher frequency to myeloid progenitors.30 In the present study, overexpression of Smad4 did not affect the lineage distribution in PB at 12 weeks after transplantation, indicating that an increase in the Smad4 level only affects self-renewal capacity and not lineage choice.
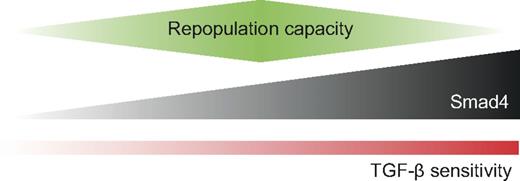
Model for the regulation of repopulative capacity by the expression level of Smad4. The precise level of Smad4 can modulate the sensitivity to TGF-β and affect the repopulative capacity of HSPCs in vivo. Smad4-deficient cells have decreased repopulative capacity and are insensitive to TGF-β. Overexpression of Smad4 leads to increased sensitivity to TGF-β, which translates into reduced repopulative capacity.
Model for the regulation of repopulative capacity by the expression level of Smad4. The precise level of Smad4 can modulate the sensitivity to TGF-β and affect the repopulative capacity of HSPCs in vivo. Smad4-deficient cells have decreased repopulative capacity and are insensitive to TGF-β. Overexpression of Smad4 leads to increased sensitivity to TGF-β, which translates into reduced repopulative capacity.
We also observed that enforced expression of Smad4 increases the sensitivity to TGF-β in colony assays in vitro. Importantly, this effect was rescued on addition of a TGF-β type I receptor inhibitor. Furthermore, Bhatia et al showed that human HSPCs express BMP receptors ALK3 and ALK6 and that BMP regulates cell proliferation and differentiation.8 However, BMP stimulation, with or without TGF-β type I receptor inhibitor, did not affect the cells, even though Smad4 is shared with the BMP signaling pathway. This result suggests that the BMP pathway is not affected by overexpression of Smad4 in HSPCs, at least not with respect to the in vitro assays performed in this study. Because our results show that the Smad4 level can modulate the response to TGF-β, it is interesting to speculate whether or not such a mechanism exists in vivo. Worthy of mention in this context is a recent publication where microRNA-mediated down-regulation of Smad4 in granulocytic precursors led to reduced sensitivity to TGF-β1.31
TGF-β has been shown to inhibit HSPC growth both by keeping them in a quiescent state of the cell cycle and by inducing apoptosis.9-12 In the present study, the transduced TGFβ-hypersensitive cells were shown to have a higher proportion of cells in the noncycling G0 phase of cell cycle. Because serum-free culture conditions were used, these data imply that the level of Smad4 is important for modulating the response to autocrine and/or paracrine TGF-β in culture. Importantly, on addition of the TGF-β type I receptor inhibitor, this effect was reversed (Figure 4B-C). Indeed, it has been shown in earlier studies that TGF-β has an autocrine effect, as addition of anti-TGF-β1 under serum-free culture conditions augmented proliferation of CD34+CD38−Lin− cells in the presence of SCF.32 Furthermore, apoptosis was increased in Smad4 vector-transduced cells. In keeping with a TGF-β–dependent mechanism, the proportion of apoptotic cells was reduced on addition of the TGF-β type I receptor inhibitor. This is in full agreement with the fact that it is the increased sensitivity to TGF-β, and not the vector itself, that is responsible for the elevated level of apoptosis in Smad4-transduced cells (Figure 4D-E). Furthermore, we performed cell cycle and apoptosis assays on transplanted cells to analyze the effect of Smad4 overexpression in HSPCs in vivo during the regeneration process. These findings parallel the in vitro data, implying that apoptosis and quiescence are increased in vivo as a consequence of enforced expression of Smad4 (supplemental Figure 2). In addition, the finding that Pu.1 is robustly down-regulated in Smad4-overexpressing cells suggests a potential molecular mechanism for the observed data, as Pu.1 deficiency in mouse models has been demonstrated to result in loss of HSC maintenance in vivo.23
The effect of TGF-β on hematopoietic cells has been well characterized, and earlier studies have shown that TGF-β1 induces CD34 antigen up-modulation.10 For this reason, we investigated whether Smad4-overexpressing cells had an increased proportion of primitive cells in vitro. However, when CD34+CD38−CD90+ CD45RA− cells were transduced, we did not observe a prolonged CD34/CD90 expression over time compared with control cells. It is possible that this is a result of increased apoptosis in culture because of the increased sensitivity to autocrine TGF-β.
In conclusion, our results demonstrate that increased Smad4 expression sensitizes human CB HSPCs to TGF-β. This leads to growth arrest and apoptosis and reduced HSPC reconstitution capacity in vivo without affecting the lineage distribution. Together, these findings show that the level of Smad4 is important for the strength of the TGF-β signal, revealing an important role for the Smad signaling pathway as a negative regulator of human HSPCs in vivo. Building on these findings, transient blocking of TGF-β signaling specifically in HSCs might be an important tool to increase reconstitution of HSCs in vivo after transplantation and for ex vivo expansion protocols that aim to increase HSC numbers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christopher Baum for the original lentiviral vector, Tariq Enver and Cristina Pina for advice and assistance with Fluidigm analysis, and Silja Andradottir for help with cord blood collection.
This work was supported by the Swedish Research Council Linnaeus (Hemato-Linné grant), the Swedish Cancer Foundation (Cancerfonden), the Swedish Cancer Society (S.K.), the Swedish Children's Cancer Society (G.K. and S.K.), the Swedish Medical Research Council (S.K.), and the Royal Swedish Academy of Sciences (Tobias Prize) financed by the Tobias Foundation (S.K.). The Lund Stem Cell Center was supported by a Center of Excellence grant in Life Sciences from the Swedish Foundation for Strategic Research.
Authorship
Contribution: E.R. designed and performed research, analyzed data, and wrote the manuscript; M.N.H. designed and performed research; U.B. and G.K. designed and performed research and contributed to writing the manuscript; and S.K. directed the research and program, designed research, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Karlsson, Molecular Medicine and Gene Therapy, Lund University Hospital, BMC A12, 22184, Lund, Sweden; e-mail: stefan.karlsson@med.lu.se; and Göran Karlsson, Molecular Medicine and Gene Therapy, Lund University Hospital, BMC A12, 22184, Lund, Sweden; e-mail: goran.karlsson@med.lu.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal