Abstract
Some women suffering from leukemia require bone marrow transplantation to be cured. Bone marrow transplantation is associated with a high risk of sterility, and some patients are offered fertility preservation by cryopreservation of the ovarian cortex. Transplantation of the ovarian cortex to women cured of leukemia who became menopausal is currently not performed because of the risk of introducing the disease. In this study, individual pieces of ovarian cortex intended for reimplantation from 25 patients with leukemia were transplanted to each of 25 nude mice for 20 weeks. The ovarian cortex was examined before and after transplantation by histology and immunohistochemistry, and RT–quantitative PCR (in the 7 patients with a known marker). Seventeen patients had the ovarian cortex retrieved when they were in complete remission. Before transplantation, 4 of 7 pieces (2 from patients in complete remission) of ovarian cortex had a positive RT–quantitative PCR. After transplantation, none of the mice revealed any sign of disease, neither in the pieces of ovarian cortex transplanted nor in any of the murine organs evaluated. Thus, the ovaries from patients in complete remission do not appear to contain viable malignant cells contrasting ovarian tissue retrieved before treatment.
Introduction
Leukemia is the most common cancer in children, and the proportion of patients surviving more than 5 years has encouragingly risen to more than 80%.1,2 Female patients receiving bone marrow transplantation usually experience severe damage to their ovaries with high risk of subsequent premature ovarian insufficiency and infertility.3,4 Consequently, the majority of patients experience difficulties in conceiving.5 Various methods to preserve fertility, such as cryopreservation of mature oocytes or embryos, have previously been attempted but cannot be applied to young girls before puberty. In addition, these methods require 2 to 4 weeks for ovarian stimulation and will invariably delay cancer treatment. In contrast, cryopreservation of the ovarian cortex can be offered on a day-to-day basis.
Worldwide, 20 healthy children have been born to women who have had frozen/thawed ovarian cortex reimplanted6-10 since the birth of the first child from this treatment in 2004.11 Although an estimated 60 women have had frozen/thawed ovarian cortex reimplanted, no women who suffered from leukemia have to our knowledge had the ovarian cortex implanted because of the risk of introducing the original cancer.
Leukemia is a disease of the blood and bone marrow, but malignant cells may be present anywhere in the body including the ovaries. Treatment usually includes an induction cure that removes most malignant cells from the blood, but may not eliminate malignant cells in the bone marrow. The ovary can be retrieved either before or after the induction cure and the timing of the retrieval may influence the risk of malignant cells in the ovaries and the quality of the oocytes in the retrieved ovarian tissue. A few studies have found traces of malignant cell markers in ovarian cortical pieces intended for fertility preservation from patients with leukemia using reverse transcription–quantitative polymerase chain reaction (RT-qPCR).12-15 In 1 study, ovarian cortex was transplanted to immunodeficient mice which resulted in 5 of 18 mice developing human leukemia with viable cancer cells.13 Ovarian metastasis was found in 8% (54 of 684) of young patients with leukemia at autopsy.16
The present study investigated the presence of malignant cells in frozen/thawed ovarian cortical tissue from patients with leukemia in a relatively large cohort of patients. The majority of the patients were in complete remission at the time of the cryopreservation of the ovarian cortex. The tissue was transplanted to immunodeficient mice for 20 weeks and was evaluated for the presence of malignant cells using histology and immunohistochemistry techniques; in the case of a known cancer marker, the transplanted ovarian tissue was evaluated with RT-qPCR.
Methods
Patients
The study population included all women in Denmark with leukemia who had 1 of their ovaries cryopreserved for fertility preservation, a total of 47 women/children diagnosed with either acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), or juvenile myelomonocytic leukemia (JMML). Of these, 9 were deceased and according to the protocol, their tissue had been destroyed. A total of 37 girls and women were invited to participate in the study (1 patient had emigrated from Denmark). Five declined to participate and 7 did not respond to letters. In total, 25 patients were included giving informed written consent after oral and written information was provided. In the case of children below 18 years, the parents signed the consent form. A total of 18 patients had received chemotherapy before removal of ovarian tissue, and 17 were in complete remission (Table 1). The study was approved by The Regional Committee on Biomedical Research Ethics (H-2-2010-096).
Patient characteristic at time of ovarian cryopreservation and total amount of ovarian cortex evaluated in the study
| Patient no. . | Age at cryopreservation, y . | Type of leukemia . | Total amount of ovarian cortex evaluated, % of 1 ovary . | Disease status at ovarian cryopreservation . |
|---|---|---|---|---|
| 1 | 15 | ALL | 9 | CR |
| 2 | 13 | CML | 18 | Chronic phase |
| 3 | 15 | ALL | 11 | CR |
| 4 | 26 | CML | 20 | Chronic phase |
| 5 | 18 | ALL | 18 | CR |
| 6 | 4 | ALL | 29 | CR |
| 7 | 21 | AML | 15 | CR |
| 8 | 3 | AML | 13 | CR |
| 9 | 23 | ALL | 9 | CR |
| 10 | 4 | ALL | 22 | CR |
| 11 | 21 | AML | 8 | CR |
| 12 | 9 | ALL | 30 | CR |
| 13 | 5 | AML | 15 | CR |
| 14 | 18 | ALL | 7 | Active phase |
| 15 | 17 | CML | 11 | Chronic phase |
| 16 | 15 | AML | 8 | CR |
| 17 | 24 | ALL | 9 | Unknown |
| 18 | 18 | AML | 9 | Active phase |
| 19 | 23 | ALL | 8 | CR |
| 20 | 2 | JMML | 18 | Active phase |
| 21 | 31 | AML | 10 | CR |
| 22 | 3 | AML | 14 | CR |
| 23 | 20 | AML | 7 | * |
| 24 | 11 | ALL | 10 | CR |
| 25 | 20 | AML | 8 | CR |
| Patient no. . | Age at cryopreservation, y . | Type of leukemia . | Total amount of ovarian cortex evaluated, % of 1 ovary . | Disease status at ovarian cryopreservation . |
|---|---|---|---|---|
| 1 | 15 | ALL | 9 | CR |
| 2 | 13 | CML | 18 | Chronic phase |
| 3 | 15 | ALL | 11 | CR |
| 4 | 26 | CML | 20 | Chronic phase |
| 5 | 18 | ALL | 18 | CR |
| 6 | 4 | ALL | 29 | CR |
| 7 | 21 | AML | 15 | CR |
| 8 | 3 | AML | 13 | CR |
| 9 | 23 | ALL | 9 | CR |
| 10 | 4 | ALL | 22 | CR |
| 11 | 21 | AML | 8 | CR |
| 12 | 9 | ALL | 30 | CR |
| 13 | 5 | AML | 15 | CR |
| 14 | 18 | ALL | 7 | Active phase |
| 15 | 17 | CML | 11 | Chronic phase |
| 16 | 15 | AML | 8 | CR |
| 17 | 24 | ALL | 9 | Unknown |
| 18 | 18 | AML | 9 | Active phase |
| 19 | 23 | ALL | 8 | CR |
| 20 | 2 | JMML | 18 | Active phase |
| 21 | 31 | AML | 10 | CR |
| 22 | 3 | AML | 14 | CR |
| 23 | 20 | AML | 7 | * |
| 24 | 11 | ALL | 10 | CR |
| 25 | 20 | AML | 8 | CR |
CR indicates complete remission; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; and JMML, juvenile myelomonocytic leukemia.
Patient had received a full cycle of intravenous chemotherapy, but was not in complete remission until after the second cycle.
Twenty patients from a previous study from our group15 were included in the present study. Six patients from the previous study did not want to participate in the present study, and 5 new patients agreed.
Ovarian tissue cryopreservation and thawing
One entire ovary was retrieved and brought to the laboratory for immediate processing or transported 4-5 hours on ice as previously described.17 The tissue was cryopreserved as previously described.17,18 In brief, the ovarian medulla, rich in blood vessel but containing few oocytes, was discarded and the ovarian cortex was isolated. The cortex was cut into pieces of approximately 1 × 5 × 5 mm. The number of pieces of ovarian cortex prepared for cryopreservation varied from 7 to 32 (median 20) depending on the size of the ovary. The 7 pieces came from a 3-year-old girl (patient 22). In the process, the cortex was rinsed several times in isotonic saline solution. The pieces of ovarian cortex were transferred to a cryoprotectant solution and equilibrated for 25 minutes on a rocking table and afterward transferred to individual ampoules with fresh cryoprotectant. The ampoules were frozen according to a slow-freeze protocol and plunged into liquid nitrogen for storage. The cortical pieces were thawed in exactly the same manner as they would have been in the case of reimplantation to a patient including 3 washing steps19 before being transplanted to immunodeficient mice or evaluated directly by RT-qPCR and histology.
Histology and immunohistochemistry
All patients had one-half or 1 entire piece of ovarian cortex examined by histology/immunohistochemistry as previously described.15 In 1 case, the parents accepted evaluation of only a single piece of ovarian cortex (patient 22) which was used in the transplantation study. Immediately after thawing, the ovarian cortex was fixed in 4% formaldehyde, processed for paraffin embedding, stained with hematoxylin and eosin (H&E) and examined by immunohistology. Before staining, the sections were heated in a microwave oven in tris-EGTA-buffer (pH 9) for 15 minutes. The staining was performed in the Techmate 500 Immunostainer, using the DAKO Envision K5007 as a secondary Ab.20 The samples were examined by 2 experienced pathologists (E.R. and E.C.-L.).
Xenotransplantation
Twenty-five female (NMRI-nu/nu) immunodeficient mice were obtained from Taconic. They were kept, 3 per cage, in individual ventilated cages with free access to food and water. Each mouse had an identification code present in the ears. The murine part of the study was approved by The Animal Experiments Inspectorate (2009/561-1590). At 9-10 weeks of age, the mice were anesthetized with isofluran (Baxter) and 1 piece of frozen/thawed ovarian cortex prepared for clinical use was transplanted to the mice. Immediately before transplanting, the pieces were divided in 2 to 4 smaller pieces and transplanted to subcutaneously prepared pockets onto both flanks of the mice. After transplantation, the mice were kept for 20 weeks and killed by cervical dislocation. The skin was visually inspected for skin metastasis and an autopsy was done. The human ovarian cortex and selected organs (ie, spleen, liver, kidney, heart, lung, and femur) were recovered, visually inspected, and fixed in 4% formaldehyde. In cases with a known molecular marker, a piece of the organs and one-half of the transplant were transferred to vials with ice-cold RNA-later (Sigma-Aldrich). After a maximum of 2 hours, the vials were frozen at −80°C.
Histology and immunohistochemistry after xenotransplantation
The transplanted ovarian tissue and the selected murine organs were embedded in paraffin. The transplant was serially sectioned into 5-μm sections, stained with H&E, and examined by immunohistochemistry using the Abs listed in Table 2. For the murine organs, a cross-section of 5 μm was made and stained with H&E. All samples were examined by 2 experienced pathologists (E.R. and E.C.-L.).
Results of histology of frozen/thawed and xenotransplanted ovarian cortex
| Patient no. . | Amount of the ovary xenotransplanted, % of 1 ovary . | Histology . | ||
|---|---|---|---|---|
| Marker . | Result, frozen/thawed cortex . | Result, xenotransplanted cortex . | ||
| 1 | 5 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 2 | 6 | CD34, CD117, MPO | Negative | Not performed |
| 3 | 5 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 4 | 7 | CD34, CD117, MPO | Negative | Negative |
| 5 | 9 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 6 | 14 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 7 | 5 | CD34, CD117, MPO | Negative | Negative |
| 8 | 7 | CD34, CD117, MPO | Negative | Negative |
| 9 | 3 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 10 | 11 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 11 | 4 | CD34, CD117, MPO | Negative | Negative |
| 12 | 10 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 13 | 8 | CD34, CD117, MPO | Negative | Negative |
| 14 | 4 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 15 | 4 | CD34, CD117, MPO | Negative | Negative |
| 16 | 4 | CD34, CD117, MPO | Negative | Negative |
| 17 | 4 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 18 | 4 | CD34, CD117, MPO | Negative | Negative |
| 19 | 4 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 20 | 9 | CD34, CD117, MPO, CD163 | Negative | Negative |
| 21 | 5 | CD34, CD117, MPO | Negative | Negative |
| 22 | 14 | CD34, CD117, MPO | Not performed | Negative |
| 23 | 4 | CD34, CD117, MPO | Negative | Negative |
| 24 | 5 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 25 | 4 | CD34, CD117, MPO | Negative | Negative |
| Patient no. . | Amount of the ovary xenotransplanted, % of 1 ovary . | Histology . | ||
|---|---|---|---|---|
| Marker . | Result, frozen/thawed cortex . | Result, xenotransplanted cortex . | ||
| 1 | 5 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 2 | 6 | CD34, CD117, MPO | Negative | Not performed |
| 3 | 5 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 4 | 7 | CD34, CD117, MPO | Negative | Negative |
| 5 | 9 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 6 | 14 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 7 | 5 | CD34, CD117, MPO | Negative | Negative |
| 8 | 7 | CD34, CD117, MPO | Negative | Negative |
| 9 | 3 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 10 | 11 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 11 | 4 | CD34, CD117, MPO | Negative | Negative |
| 12 | 10 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 13 | 8 | CD34, CD117, MPO | Negative | Negative |
| 14 | 4 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 15 | 4 | CD34, CD117, MPO | Negative | Negative |
| 16 | 4 | CD34, CD117, MPO | Negative | Negative |
| 17 | 4 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 18 | 4 | CD34, CD117, MPO | Negative | Negative |
| 19 | 4 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 20 | 9 | CD34, CD117, MPO, CD163 | Negative | Negative |
| 21 | 5 | CD34, CD117, MPO | Negative | Negative |
| 22 | 14 | CD34, CD117, MPO | Not performed | Negative |
| 23 | 4 | CD34, CD117, MPO | Negative | Negative |
| 24 | 5 | CD34, TdT, CD79a, CD3 | Negative | Negative |
| 25 | 4 | CD34, CD117, MPO | Negative | Negative |
CD indicates cluster of differentiation; MPO, myeloperoxidase; and TdT, terminal deoxynucleotidyl transferase.
Quantitative polymerase chain reaction
In a previous study, a piece of ovarian cortex had been evaluated by RT-qPCR in case of a known molecular marker.15 In the present study, another piece of ovarian cortex was evaluated after 20 weeks of xenotransplantation. After recovery from the mice, one-half of the transplant was assigned to RT-qPCR and stored at −80°C in RNA-later (Sigma-Aldrich). On thawing, tissue samples were homogenized in 300 μL of buffer RLT (QIAGEN) containing 40mM dithiothreitol (DTT; Applichem) using a TissueRuptor (QIAGEN) and disposable probes. RNA isolation was performed using the RNeasy Fibrous Tissue Mini Kit (QIAGEN) according to the manufacturer's instructions, and RNA purity and yield was determined on a NanoDrop ND-1000 spectrophotometer. Reverse transcription was done using SuperScript VILO (Invitrogen), and the cDNA was subsequently diluted 4-fold in water and used immediately or stored at −80°C. Quantitative PCR was done as previously described15 with minor modifications to the TEL-AML1/ETV6-RUNX121 and Abelson (ABL1)22 assays. Appropriate positive and negative controls were included. All data were evaluated qualitatively using ABL1 transcript levels as indicators of RNA quality and quantity. The detection limit of the assays was at threshold cycle (Ct) 40.
Follicle density
Before freezing, a small piece (∼ 2 × 2 × 1 mm) of ovarian cortex was fixed in Bouin solution in 17 patients. The piece was processed for histology, sliced into 30-μm sections, and stained with periodic-acid Schiff reagents and Mayer hematoxylin. The follicular density was determined as previously described.23
The patients who had received chemotherapy before excision of the ovary were compared with a group of women who had not received chemotherapy before excision of the tissue. Linear regression analysis was used to evaluate a possible relationship between the age and follicle density (log-transformed). P < .05 was considered statistically significant.
Results
Xenotransplantation
Twenty-five mice underwent transplantation with ovarian cortex from 25 individual patients with leukemia and only 3 were derived from patients in the active phase of leukemia. All 25 mice lived for the duration of the 20-week study period and none of them developed any macroscopic signs of malignancy, but 2 showed an enlarged spleen at autopsy. The majority of transplanted ovarian cortex were clearly vascularized with obvious blood supply and had survived the transplantation procedure. All pieces of transplanted ovarian cortex were reduced in size during the transplantation period. One piece of ovarian cortex could not be found on autopsy possibly because of reabsorption of the transplant, (patient 2), and in 1 case the retrieved transplant did not contain ovarian tissue (patient 9).
Histology
Using the Abs listed in Table 2 and appropriate positive and negative controls, 2 experienced pathologists (E.C.-L. and E.R.) found no malignant cells in the ovarian cortex neither before nor after transplantation. Murine organs were evaluated in a similar way and showed no signs of invasion of leukemic cells. The histology of the 2 enlarged spleens was normal. The presence of follicles after the transplantation period was confirmed in several of the grafts (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
RT-qPCR analysis
The ovarian cortex showed a positive RT-qPCR result in 4 of the 7 patients with a known molecular marker before transplantation.15 Two positive results came from patients in the chronic phase and 2 from patients in complete remission. No patients with leukemia in the active phase had a molecular marker. The threshold cycle (Ct) of the fusion transcripts was high and close to the limit of detection (Ct = 40; Table 3). The cortex was destroyed in the process and another piece from the same patient was transplanted to a nude mouse. After 20 weeks of xenotransplantation, one-half of each of the transplanted pieces of cortex was again examined by RT-qPCR and no disease-specific molecular markers were detected in any of the transplanted tissue, including cases found to be positive in the previous study. One sample was not analyzed as the transplanted ovarian tissue was not recovered from the mouse; in another, the amount of RNA isolated was too small to be analyzed.
Results of RT-qPCR of frozen/thawed ovarian cortex
| Patient . | Fusion transcript, Ct . | Control transcript, Ct . | RNA concentration at extraction, ng/μL . | Amount of the ovary evaluated by RT-qPCR, % of 1 ovary . | Result . |
|---|---|---|---|---|---|
| Results before transplantation to nude mice | |||||
| 2 | 37.4 | 24 | 264 | 3 | Positive |
| 38.1 | 25 | 64 | 6 | ||
| 4 | 38.4 | 24 | 175 | 3.5 | Positive |
| Undetectable | 27 | 28 | 7 | ||
| 6 | 34.3 | 23 | 78 | 7 | Positive |
| Undetectable | 23 | 78 | |||
| 7 | Undetectable | 24 | 60 | 2.5 | Positive |
| 38.2 | 21 | 219 | 5 | ||
| 9 | Undetectable | 23 | 387 | 3 | Negative |
| 12 | Undetectable | 23 | 20 | 5 | Negative |
| Undetectable | 26 | 23 | 10 | ||
| 15 | Undetectable | 29 | 9 | 2 | Negative |
| Undetectable | 23 | 89 | 4 | ||
| Results after transplantation to nude mice | |||||
| 2 | Not performed | Not performed | Not performed | None left | NA |
| 4 | Undetectable | 26 | 12 | 3 | Negative |
| 6 | Undetectable | 27 | 10 | 7 | Negative |
| Undetectable | 28 | 10 | |||
| 7 | Undetectable | 24 | 18 | 2.5 | Negative |
| Undetectable | 23 | 36 | |||
| 9 | Undetectable | Undetectable | 1 | 1.5 | NA |
| 12 | Undetectable | 26 | 10 | 5 | Negative |
| Undetectable | 27 | 3 | |||
| 15 | Undetectable | Undetectable | 18 | 2 | Negative |
| Undetectable | 24 | 35 |
| Patient . | Fusion transcript, Ct . | Control transcript, Ct . | RNA concentration at extraction, ng/μL . | Amount of the ovary evaluated by RT-qPCR, % of 1 ovary . | Result . |
|---|---|---|---|---|---|
| Results before transplantation to nude mice | |||||
| 2 | 37.4 | 24 | 264 | 3 | Positive |
| 38.1 | 25 | 64 | 6 | ||
| 4 | 38.4 | 24 | 175 | 3.5 | Positive |
| Undetectable | 27 | 28 | 7 | ||
| 6 | 34.3 | 23 | 78 | 7 | Positive |
| Undetectable | 23 | 78 | |||
| 7 | Undetectable | 24 | 60 | 2.5 | Positive |
| 38.2 | 21 | 219 | 5 | ||
| 9 | Undetectable | 23 | 387 | 3 | Negative |
| 12 | Undetectable | 23 | 20 | 5 | Negative |
| Undetectable | 26 | 23 | 10 | ||
| 15 | Undetectable | 29 | 9 | 2 | Negative |
| Undetectable | 23 | 89 | 4 | ||
| Results after transplantation to nude mice | |||||
| 2 | Not performed | Not performed | Not performed | None left | NA |
| 4 | Undetectable | 26 | 12 | 3 | Negative |
| 6 | Undetectable | 27 | 10 | 7 | Negative |
| Undetectable | 28 | 10 | |||
| 7 | Undetectable | 24 | 18 | 2.5 | Negative |
| Undetectable | 23 | 36 | |||
| 9 | Undetectable | Undetectable | 1 | 1.5 | NA |
| 12 | Undetectable | 26 | 10 | 5 | Negative |
| Undetectable | 27 | 3 | |||
| 15 | Undetectable | Undetectable | 18 | 2 | Negative |
| Undetectable | 24 | 35 |
Ct indicates threshold cycle; RT-qPCR, reverse transcription–quantitative polymerase chain reaction; and NA, not available.
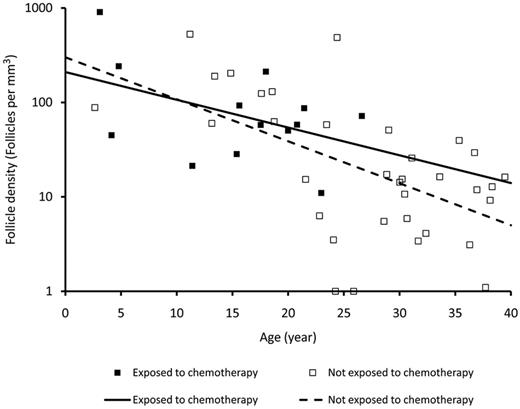
Follicle density
Follicular density declined with age. The 2 linear regression lines obtained from patients with or without being exposed to chemotherapy prior to excision of the ovarian tissue were not significantly different (Figure 1).
Follicle density in ovarian cortex in relation to age and exposure to chemotherapy. Follicle density in fresh human ovarian cortex from patients with leukemia exposed to chemotherapy before retrieval of the tissue and women not exposed to chemotherapy before retrieval of the tissue. The follicle density declines with age, but no difference is seen between patients who have received first line of chemotherapy and women who have not received chemotherapy.
Follicle density in ovarian cortex in relation to age and exposure to chemotherapy. Follicle density in fresh human ovarian cortex from patients with leukemia exposed to chemotherapy before retrieval of the tissue and women not exposed to chemotherapy before retrieval of the tissue. The follicle density declines with age, but no difference is seen between patients who have received first line of chemotherapy and women who have not received chemotherapy.
Discussion
The present study was unable to show malignant cell contamination in frozen/thawed ovarian cortex intended for transplantation from each of 25 patients with leukemia after 20 weeks of transplantation to immunodeficient mice. This study mainly evaluated leukemic patients scheduled for allogenic bone marrow transplantation. This specific group of patients bears a very high risk of becoming infertile, contrasting the patients receiving the firstline treatment who have a low risk of infertility.24 Furthermore, most of the patients were in complete remission after a few rounds of chemotherapy, which appeared not to reduce the follicular density as measured in the present study.
In addition, the patients actually survived the disease (using the tissue of diseased patients is not allowed according to Danish legislation) and represent those with a better prognosis. Collectively, the results of the present study suggest that ovarian tissue from surviving leukemic patients in complete remission at the time of excision is without viable malignant cells.
This conclusion is not in line with our previously published results,15 where small traces of disease-specific fusion transcripts were found in the ovarian cortex before transplantation as evaluated by RT-qPCR. However, the fusion transcripts were detected near the limit of detection of the assays, indicating that only a few cells were positive for the fusion transcripts. Indeed, the cautious interpretation of the results at that time was to suggest that ovarian tissue may contain malignant cells, but further studies were required to demonstrate whether they were viable.15 The present study addressed this question and found that the RT-qPCR results from the previous study could not be repeated in the tissue after transplantation. However, a smaller amount of tissue was available resulting in lower sample-specific assay sensitivity. Nevertheless, the fact that no malignant cells were detected by any of the methods applied in this study indicates that the few malignant cells potentially present before transplantation were either not viable or too few to cause a relapse during transplantation.
If viable malignant cells had been present in the transplanted ovarian cortex, we would expect them to proliferate and manifest in the immunodeficient mice during the 20-week transplantation period as human tumors that do grow on mice normally start to grow fast and waiting more than 20 weeks does not increase the numbers substantially.25
The present study contrasts results from a recent study by Dolmans et al, in which contamination to the ovarian cortex was demonstrated by presence of leukemic cells in immunodeficient mice transplanted with ovarian cortex from patients with leukemia in 5 of 18 patients.13 However, in the present study, 17 of 21 patients with acute leukemia were in complete remission at the time of retrieval of the ovary whereas only 2 patients of 12 with acute leukemia from Dolmans' study had received a full cycle of intravenous chemotherapy before excision of ovarian cortex. The mice with ovarian cortex from the 2 patients who had received intravenous chemotherapy failed to develop leukemia in the Dolmans study. It is reasonable to assume that tissue from a patient in complete remission is less likely to harbor malignant cells than tissue from a patient in the active phase. Our results suggest that if the ovarian cortex is collected during complete remission a single piece of ovarian cortex contains no or too few malignant cells to cause disease in mice even though the RT-qPCR was positive in 2 patients in complete remission.
In the Dolmans study, 5 of 10 mice did not develop leukemia after transplantation with ovarian cortex from patients with acute leukemia in the active phase.13 In the present study, only 2 patients (patients 14 and 18) had acute leukemia in the active phase and none of the mice transplanted with tissue from these patients developed leukemia. When these mice do not develop leukemia after transplantation, it is probably because the tissue does not contain viable cells. However, the mice model is not perfect although it has proved a good model to detect the presence of human leukemic cells.13 Currently, this model is probably the most sensitive to detect the presence of viable malignant cells in frozen/thawed human ovarian tissue although using immunodeficient mice as a bioincubator for the development of leukemia cells may have some drawbacks. The transmission of rat leukemia requires very few malignant cells (20-200),26,27 but it is less clear how many human cells are needed to introduce leukemia in mice28,29 and it also depends on the mouse strain.30 The administration route affects the take rate and Imamura et al found a higher take rate for human leukemic cell lines after subcutaneous inoculation as performed in the present study compared with intraperitoneal inoculation.31
The next question is how many cancer cells are capable of introducing leukemia. There is no excess risk of cancer-inclusive leukemia after blood transfusion from donors diagnosed with cancer within 5 years of the blood donation32 and the rate of donor-related malignancies after organ transplantation is very low,33 but a donor-derived leukemia has been seen 2 years after transplantation.34 If few viable leukemic cells were present in the remaining pieces of ovarian cortex intended for reimplantation, the graft-versus-leukemia effect might benefit the patient35 as most of our patients have received allogeneic bone marrow transplantation and thus have donor-derived T cells.
As the ovaries are retrieved in complete remission, the patients have received chemotherapy but the induction cure of AML and ALL generally does not include alkylating agents2,36,37 and the treatment of ALL and AML without bone marrow transplantation is considered to bear a low risk of infertility.24 Data from the Childhood Cancer Survivor Study showed no difference in timing of menarche among survivors of ALL treated with chemotherapy alone compared with siblings38 and a very low risk (3%) of acute ovarian failure and premature menopause among all survivors of leukemia.39,40
Transplantation of frozen/thawed ovarian tissue from a patient who had received chemotherapy before excision of the tissue has restored ovarian function for more than 5 years and resulted in live birth.41,42 Furthermore, this illustrates that follicles and oocytes survived the chemotherapy, and the possible period of cryostorage and transplantation, appears to have a developmental potential that is not compromised, confirming earlier studies on patients exposed to irradiation.
Alternatively, if primordial follicles could be grown and matured in vitro or isolated follicles could be transplanted, the risk of introducing a cancer cell could be avoided. They could represent new methods that could be used to save fertility. These methods have produced live offspring in mice43 and mature oocytes in primates44 but have not been developed to a stage that permits use in humans despite an intense research effort. However, many patients with leukemia are young, and several decades may elapse before they actually require having restored fertility. In our opinion, patients with leukemia should be offered fertility preservation before starting treatment like bone marrow transplantation with a high risk of damaging ovarian function.
In conclusion, the present study did not find any signs of viable malignant cells in pieces of ovarian cortex from each of 25 patients suffering from leukemia with the majority in complete remission. However, the ovarian cortex showed a positive RT-qPCR result in 4 of the 7 patients with a known molecular marker before transplantation; it cannot be excluded that cancer cells may exist in other pieces of ovarian cortex not evaluated. Although the present study contained a relatively large number of patients, additional studies showing similar results are desirable before transplantation into patients is considered. The present results support that there may be a way to salvage the fertility of girls and young women with leukemia undergoing gonadotoxic treatment.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
Some data presented in abstract form at the 2nd World Congress on Fertility Preservation, Miami Beach, FL, December 8-10, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The technical laboratory assistance of Tiny Roed and Inga Husum is gratefully acknowledged.
This work was supported by unrestricted grants from the Danish Cancer Foundation (DP05112/ R2-A41-09-S2), the Danish Medican Research Council (271-07-0452; 09-072265), the Novo Nordic Foundation, Sophus Carl Emil Friis and wife Olga Doris Friis' Foundation, The Lundbeck Foundation, and the University Hospital of Copenhagen. They had no role in gathering, analyzing, or interpreting data.
T.G. is a PhD candidate at Copenhagen University and this work is submitted in partial fulfillment of the requirement for the PhD.
Authorship
Contribution: T.G. was the principal investigator, conducted the study, performed the xenotransplantations, and wrote the manuscript; E.C.-L. and E.R. examined the histology; M.T.A. performed RT-qPCR, evaluated the results, and participated in writing the manuscript; M.K.A. identified patients with markers for qRT-PCR, evaluated results, and reviewed the manuscript; S.D.S. measured the follicle density; M.R. evaluated results and reviewed the manuscript; and C.Y.A. was the principal investigator and participated in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tine Greve, Laboratory of Reproductive Biology, Rigshospitalet–Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, Afsnit 5712, DK-2100 Copenhagen, Denmark; e-mail: tinegreve@gmail.com.