In this issue of Blood, Li and colleagues demonstrate the requirement for T cells in mediating the action of parathyroid hormone (PTH) treatment to expand primitive hematopoietic stem/progenitor cells (HSPCs), highlighting a complex interaction among cells of the immune system, bone and the HSPCs.1
Since the development of the niche hypothesis for the hematopoietic stem cell (HSC) by Schofield,2 numerous studies have been performed to improve our understanding of the extrinsic regulators of stem cell number and function. Based on the early observations that CFU-S are present in higher numbers near the endosteal bone surface, Taichman and colleagues hypothesized that bone-forming osteoblasts may be the critical cellular component that supports HSCs in vivo.3 To further support the localization of primitive HSCs in the endosteum, Nilsson and colleagues demonstrated that a primitive population of HSCs preferentially localized to the endosteal region after transplantation.4 Taken together, these 2 studies suggested that the endosteal region and osteoblastic lineage cells comprise the microenvironmental niche for the HSC in vivo. However, direct in vivo evidence for the role of osteoblasts in HSC regulation and maintenance came from studies in which osteoblast numbers were manipulated experimentally.5,6 In one of these studies, osteoblast-specific expression of a constitutively active form of parathyroid hormone 1 receptor (PTHR1), the common receptor for parathyroid hormone (PTH) and PTH-related protein (PTH-rP), was achieved using the type 1 collagen α1 (Col1a1) promoter, which is specific to maturing and mature osteoblasts.5 These mutant mice displayed simultaneous increase in the number of both osteoblasts and HSCs in the BM.
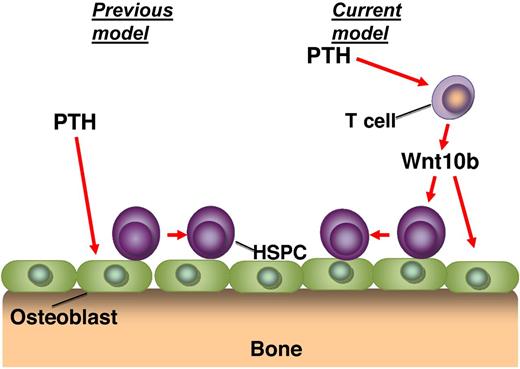
This observation led to further studies of the use of PTH as an approach to therapeutically target the HSCs via the niche.7 It has been generally assumed that the mechanism of action in these studies was through a direct stimulation of the osteoblastic cells by PTH, which in turn led to an increase in the number or function of HSPCs. However, this study by Li et al suggests that there is another critical player in this pathway.1 These observations build on previous studies from the same group that has demonstrated the absolute requirement of PTH signaling in T cells to be able to mediate the actions of PTH on bone.8 Specifically, they demonstrated that the expression of Wnt10b after stimulation of T-cells with intermittent PTH (iPTH) was required for the anabolic action of the treatment. Here Li et al use a variety of genetic knockout mice to similarly show that iPTH treatment does not expand the HSPC population in mice that are deficient in T cells, or in mice that have T cells that are lacking the PTH receptor. Extending these studies to examine the mechanism of action, the role of the Wnt10b protein secretion from the T cells was also shown to be crucial for the action of iPTH (see figure). Further, it was clearly demonstrated that an up-regulation of Wnt responsive genes in the bone marrow stromal cells and HSPCs is dependent on T cells and Wnt10b expression. Interestingly, stimulation of the HSPC population using iPTH treatment leads to an expansion of the ST-HSC population, without any effect on the LT-HSC population, suggesting that there may be separate mechanisms of niche regulation for these distinct subsets of the HSPCs.
The proposed mechanism of action of iPTH treatment on hematopoietic stem/progenitor cells (HSPCs). Previous studies have suggested that parathyroid hormone (PTH) acts directly on the osteoblasts to mediate the expansion of the HSPC pool (previous model). The study demonstrates that PTH acts to stimulate T cells, which release Wnt10b, which then acts on the osteoblasts and HSPCs (current model).
The proposed mechanism of action of iPTH treatment on hematopoietic stem/progenitor cells (HSPCs). Previous studies have suggested that parathyroid hormone (PTH) acts directly on the osteoblasts to mediate the expansion of the HSPC pool (previous model). The study demonstrates that PTH acts to stimulate T cells, which release Wnt10b, which then acts on the osteoblasts and HSPCs (current model).
This very elegant demonstration that T cells are absolutely required to mediate the action of PTH to stimulate the expansion of primitive hematopoietic cells may also have implications in other areas of stem cell biology. For many years it has been known that T cell–depleted autologous and allogeneic transplants result in lower levels of engraftment during the first 6 months after transplantation. This implies that T cells facilitate optimal engraftment of CD34+ cells and has been supported by clinical trials in humans demonstrating that presence of CD8+ cells, but not CD4+ cells, results in an increased level of engraftment in the allogeneic transplant setting.9 The mechanism of action by which CD8+ cells facilitate engraftment is generally attributed to the elimination of residual immune cells of the recipient. However, stem cells transplanted into multi-immunodeficient hosts similarly benefit from accessory cells, suggesting the possibility of other roles often assumed to be that of cytokine production.10 This study by Li and colleagues highlights a further interaction between cells of the immune system and the HSPCs through the Wnt pathway, which may provide clues to the mechanisms of action between stem cells and T-cells in other settings.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal