Abstract
Abstract 966
of this analysis was to provide long-term follow-up of the GCLLSG CLL3X trial which aimed at evaluating reduced-intensity HSCT in patients with poor-risk CLL, and to compare the impact of the newly identified risk factors SF3B1 and NOTCH1 mutations on the outcome of HSCT with that of established prognostic factors, such as TP53 abnormalities.
CLL3X included 100 patients with poor-risk CLL (median age 53 (27-65) years), of whom 90 patients were allografted with blood stem cells from related (40%) or unrelated donors (60%). 24% had refractory CLL at HSCT, and 13% received in-vivo T cell depletion (TCD) with alemtuzumab during conditioning. All genetic studies were performed in the Ulm central reference laboratory of the GCLLSG. Genomic aberrations and IGHV mutation status were analyzed by FlSH and direct sequencing as previously described. NOTCH1 was studied by direct sequencing of a PCR fragment from the PEST domain (exon 34, chr9:139,390,619-139,391,290). SF3B1 (exons 13–16) and TP53 (exons 4–10) were analyzed by DHPLC (WAVE® 3500HT, Transgenomic Inc) with subsequent sequencing of aberrant fragments, if needed after subcloning.
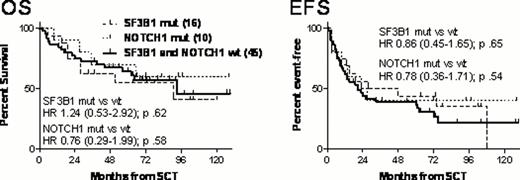
With a median follow-up of 6.0 (0.6-10.8) years, 6-year overall survival (OS) of all 100 patients enrolled was 53%. The 90 allografted patients had a 6-year OS and event-free survival (EFS) of 58% and 38%, respectively. Of 28 patients who were relapse-free and MRD-negative 12 months after HSCT, only 3 subsequently experienced clinical disease recurrence at 2.8, 5.0, and 9.0 years post transplant, and 24 (86%) remained MRD-negative throughout the whole follow-up. SF3B1, NOTCH1, and TP53 mutational status could be obtained each in 80/100 patients, and 17p FISH results were available in 82/100 patients. SF3B1 abnormalities were found in 26%, NOTCH1 abnormalities in 14%, and TP53 mutations in 30% of patients; whereas 17p- was detected in 20% of patients with diagnostic material available. Whilst SF3B1 and NOTCH1 mutations were mutually exclusive, 29% of patients with TP53 mutations also had an SF3B1 or NOTCH1 abnormality, and 46% of TP53-mutated patients had a concomitant 17p deletion. By univariate comparisons, we did not find significant survival differences between SF3B1-mutated, NOTCH1-mutated and SF3B1+NOTCH1 wild-type patients (Figure). Multivariate analysis using Cox regression modeling did not show a significant impact of SF3B1, NOTCH1, and TP53 (TP53mut and/or 17p-) abnormalities on OS and EFS (Hazard ratios (HRs) of SF3B1mut 0.82, 95%CI 0.35–1.97 and 0.64, 95%CI 0.32–1.31; HRs of NOTCH1mut 0.69, 95%CI 0.22–2.12 and 0.73, 95%CI 0.29–1.81; HRs of TP53abn 0.99, 95%CI 0.42–2.32 and 0.61, 95%CI 0.31–1.21). In contrast, TCD and refractory disease at HSCT retained their adverse impact on OS and EFS as already observed in an earlier analysis at a follow-up time of 3.8 years (Blood Oct 7, 2010).
HSCT can provide long-term EFS in approximately 40% of patients with poor-risk CLL. Patients who are in MRD-negative remission one year after HSCT have a >80% probability of durably remaining in this status. Long-term disease control does not appear to be affected by the presence of SF3B1, NOTCH1, and TP53 abnormalities, suggesting that HSCT can overcome the treatment resistance found to be associated with this abnormality.
Hallek:Roche: Consultancy, Honoraria, Research Funding. Stilgenbauer:Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal