Abstract
Abstract  963
963
We showed previously that a transplant conditioning regimen of treosulfan combined with fludarabine was associated with low non-relapse mortality (NRM) and a high probability of survival in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) with low or intermediate risk cytogenetics (Nemecek et al, BBMT 2011). Relapse rates were high, however, in patients with high-risk cytogenetics, which resulted in a 2-year relapse-free survival of 39% in this group.
The objective of the present study was to reduce the relapse incidence and improve overall survival (OS) in patients with high-risk AML or MDS by adding low-dose TBI (2 Gy) to the treosulfan plus fludarabine regimen.
Ninety-six patients with AML (n=60) or MDS (n=36) were enrolled. Patients were 60 years of age or younger (median age: 50 years) and had high-risk MDS, unfavorable or intermediate-risk AML in first complete remission (CR) or any AML beyond first CR. Cytogenetic risk in AML was stratified using the Southwest Oncology Group criteria, while the World Health Organization-based Prognostic Scoring System was used in patients with MDS. Patients received intravenous (IV) treosulfan, 14 g/m2/day on days -6 to -4, IV fludarabine 30 mg/m2/day on days -6 to -2, and 2 Gy TBI on day 0, followed by infusion of marrow (n=11) or peripheral blood stem cells (n=85) from related (n=27) or unrelated (n=69) donors. Donors were single HLA allele-level mismatched in 9 patients and HLA-matched at the allele level in 87 patients. Graft-versus-host disease (GVHD) prophylaxis consisted of methotrexate (10 mg/m2 on days +1, +3, +6 and +11 post-HCT) and tacrolimus (days –1 to +180).
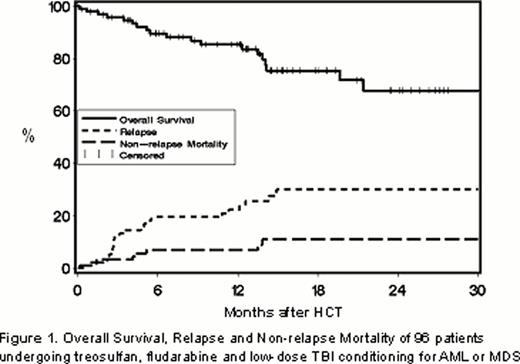
All evaluable patients engrafted. With a median follow-up of 12.8 (range, 0.2 – 34.7) months in surviving patients, the estimated 2-year progression-free survival and OS were 59% and 68%, respectively. The cumulative incidences of relapse or progression and NRM at 2 years were 30% and 11%, respectively (Figure 1). The incidences of grade II (grades III-IV) acute and extensive chronic GVHD at 2 years were 48% (11%) and 40%, respectively.
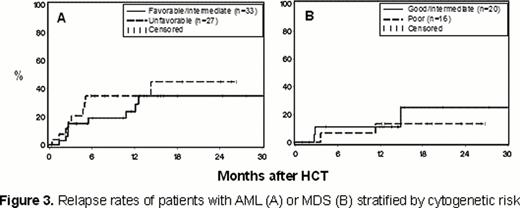
Relapse occurred in 39% of patients with AML and 18% of patients with MDS (Figure 2A). OS was 61% among patients with AML and 79% in patients with MDS (Figure 2B). In univariate analyses, cytogenetic risk status did not impact relapse for the entire cohort nor for patients with AML or MDS analyzed separately (Figures 3A and 3B). As a result, 2-year OS rates were similar in patients with favorable/intermediate and unfavorable cytogenetic risk AML (64% and 57%, respectively) or in good/intermediate and poor risk MDS (88% and 71%, respectively). Patients with AML with minimal residual disease (MRD; n=10) or in refractory relapse (n=4) at the time of HCT, had higher relapse rates (87% vs. 25%) and lower OS (25% vs. 81%) at 2 years than patients in remission without MRD at the time of HCT.
In summary, treosulfan, in combination with fludarabine and low-dose TBI was an effective conditioning regimen for allogeneic HCT in patients with AML (in CR at the time of HCT) or MDS. Relapse and OS rates in patients with high-risk cytogenetics did not differ significantly from those in patients with low or intermediate risk karyotypes. The relapse rate was particularly low in patients with MDS, including those with high risk cytogenetics, and further trials with treosulfan-based regimens are warranted. For patients with AML who have residual disease at HCT novel regimens need to be developed. The data suggest that in patients with AML high risk as defined by cytogenetics had a different impact on outcome than high risk as defined by other parameters, such as tumor burden.
Gyurkocza:medac GmbH, Hamburg, Germany: Research Funding. Off Label Use: Off-label usage of treosulfan, fludarabine, methotrexate and tacrolimus will be discussed. Sickle:medac GmbH, Hamburg, Germany: Research Funding. Baumgart:medac GmbH, Hamburg, Germany: Employment. Deeg:medac GmbH, Hamburg, Germany: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal