Abstract
Abstract 944
MM-009/010 established lenalidomide (LEN) + dexamethasone (DEX) as a standard of care in the treatment (Tx) of relapsed/refractory multiple myeloma (RRMM; Dimopoulos, NEJM. 2007; Weber, NEJM. 2007). The greatest benefits were observed when LEN + DEX was used at first relapse (Stadtmauer, Eur J Haematol. 2009). MM-015 is a pivotal, double-blind, randomized, placebo-controlled phase 3 trial comparing the efficacy and safety of melphalan-prednisone-lenalidomide followed by lenalidomide maintenance (MPR-R) with fixed-cycle melphalan-prednisone (MP) and melphalan-prednisone-LEN (MPR) in elderly patients (pts) with newly diagnosed multiple myeloma (NDMM) ineligible for autologous stem-cell transplant. The final analysis demonstrated unprecedented improvement in progression-free survival (PFS) in pts receiving MPR-R vs MP (31 vs 13 months [mos]; P < 0.001) with manageable toxicity (Palumbo, NEJM. 2012). The aim of this post-hoc analysis was to assess post-progression outcomes by second-line Tx and by initial MM-015 Tx arm (MPR-R, MPR, and MP).
Induction and maintenance Tx has been described (Palumbo, NEJM. 2012). Pts with progressive disease during MM-015 could enroll in an open-label extension phase (OLEP) to receive LEN 25 mg (D1-21) ± DEX 40 mg (D1-4, 9–12, and 17–20) or could receive any other anti-myeloma Tx outside of the protocol. This analysis includes data up to Apr 10, 2012 (median follow-up: 48 mos after initial randomization). Time to progression (TTP) was assessed from randomization to disease progression as assessed by investigator. Time from start of second line to start of third-line Tx was assessed as a surrogate for TTP in second line. Safety data were assessed only for pts enrolled in the OLEP.
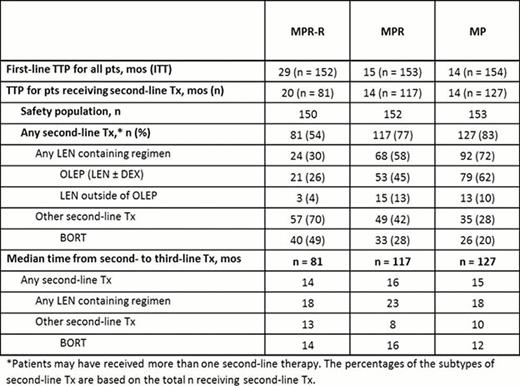
A total of 459 pts were enrolled in MM-015. Consistent with the superior PFS of MPR-R, fewer pts from the MPR-R arm progressed compared with the MPR and MP arms: 54% (81/150) vs 77% (117/152), and 83% (127/153) respectively. Compared with the overall population, pts receiving second-line Tx had shorter median first-line TTP (29 vs 20 mos), particularly for the MPR-R arm. This suggests that the present subset of pts in the MPR-R arm who started second-line therapy represents the early progressors, those with worse prognosis or more aggressive disease (Table). More pts received second-line Tx in the MP (72%) and MPR (58%) arms vs MPR-R arm (30%, Table); second-line Tx type was heterogeneous for MPR-R pts. Median time from second- to third-line Tx was significantly longer for LEN-based Tx vs non-LEN-based Tx across the 3 arms: MPR-R (18 vs 13 mos; P = 0.044), MPR (23 vs 8 mos; P = 0.02), and MP (18 vs 10 mos; P = 0.001). Median time from second- to third-line therapy with bortezomib (BORT)-based regimens was 14, 16, and 12 mos, respectively. This corresponded to higher proportions of pts remaining on second-line LEN at 2 yrs (38%, 44%, and 40%) vs non-LEN (15%, 30%, and 13%) for MPR-R, MPR, and MP, respectively. When evaluating second-line BORT, 22%, 33%, and 17%, respectively, had not progressed from second- to third-line therapy at 2 years. Prior LEN maintenance did not appear to induce resistant relapses as time from second- to third-line Tx was similar for all arms (Table) and all comparisons: MPR-R vs MP (hazard ratio [HR] = 0.924; P = 0.69), MPR-R vs MPR (HR = 1.076; P = 0.71), and MPR vs MP (HR = 0.895; P = 0.53). With limited follow-up, no significant differences in post-relapse OS have been detected. Newly occurring grade 3/4 adverse events (AEs) reported for ≥ 5% of pts entering the OLEP (n = 153) were neutropenia (11%) and anemia (5%). Grade 3/4 deep vein thrombosis and peripheral neuropathy occurred in 3% and 1% of pts, respectively.
LEN-based Tx was active in the second line, with comparable efficacy regardless of first-line therapy (MPR-R, MPR, or MP). Although pt numbers are relatively small, and this is a non-randomized comparison, results with second-line LEN-based therapy compared favorably to outcomes with other Tx. The OLEP tolerability profile was favorable, with limited newly occurring grade 3/4 AEs. Importantly, LEN maintenance does not appear to induce resistant relapses. These results support the known activity of LEN as second-line MM therapy.
Dimopoulos:Celgene Corp: Honoraria. Off Label Use: Lenalidomide as frontline treatment of multiple myeloma. Petrucci:Celgene Corp: Honoraria. Foa:Celgene Corp: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Catalano:Celgene Corp: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Yu:Celgene Corp: Employment, Equity Ownership. Herbein:Celgene Corp: Employment, Equity Ownership. Jacques:Celgene Corp: Employment, Equity Ownership. Palumbo:Celgene Corp: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal