Abstract
Abstract  875
875
Imatinib treatment has radically changed the prognosis of patients with chronic myeloid leukaemia (CML). However, around 23–32% of patients discontinue this therapy due to lack of efficacy, so there is scope to further improve the management of CML patients. We sought to identify a biomarker, or biomarkers that could be used to predict at diagnosis which patients will respond to first line treatment using routinely accessible material.
TaqMan Low Density Array (TLDA) technology was used to measure the expression of 29 genes that have been previously implicated in CML pathogenesis, normalized against 3 control genes (GUSB, B2M and 18S). RNA was extracted from archived white cell lysates from the peripheral blood of 73 patients (with appropriate ethical approval), converted to cDNA via first-strand synthesis and these were then analyzed as the learning cohort. ROC curves were plotted in order to find the optimal thresholds for each gene to divide patients into low and high expression groups; in the case of a low area under curve, the median expression value was used. Imatinib failure (IF) was defined as loss of complete hematologic response (CHR) or of complete cytogenetic response (CCyR), progression to advanced phase disease, death or change in treatment from imatinib due to lack of efficacy. Imatinib failure free survival (IFFS), cumulative incidence of CCyR and overall survival related to CML (CML OS) were analyzed by Kaplan-Meier and log-rank test for each gene. Results were validated in an independent cohort composed of 56 patients enrolled in the SPIRIT-1 trial.
Of the 29 genes, only PTCH1 (inhibitor of Smoothed (SMO), member of the Hedgehog (Hh) signalling pathway and tumour suppressor gene involved in different cancers) and XIAP (X-linked inhibitor of apoptosis) were significant across multiple endpoints across both cohorts. Using a consistent PTCH1 expression threshold, significant differences in IFFS, CCyR and CML OS were seen, while XIAP predicted only for IFFS and CCyR.
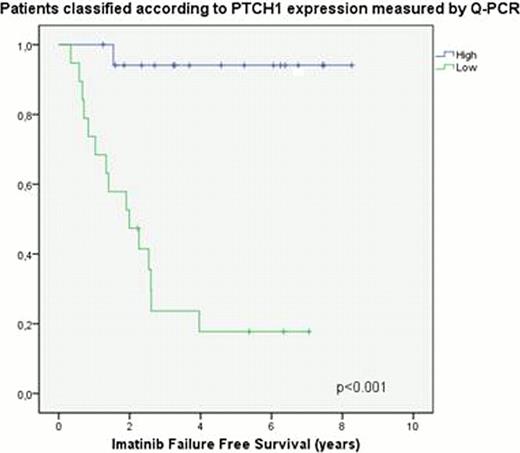
In order to corroborate our results, RT-qPCR using a different set of primers and probes (designed in-house) was performed on a subset of 37 patient diagnostic samples from the initial cohort and a further 20 samples taken on treatment, once patients had achieved CCyR, were also analysed for comparison. PTCH1 was successfully validated, and there was good correlation (r=0.823, p<0.001) between both techniques; however, the RT-qPCR results for XIAP did not recapitulate the TLDA data. Low and high PTCH1 expression groups (measured by RT-qPCR) had a 5 year rates of IFFS of 18% vs 94% (p<0.001), CCyR of 46.1% vs 100% (p=0.005) and OS CML 74% vs 100% (p=0.017). Cox regression using PTCH1 expression (categorised into high/low using the ROC), age and Sokal score demonstrated that PTCH1 expression was an independent predictor of IFFS and CCyR, irrespective of the method used for quantification. PTCH1 measurement by RT-qPCR using the second primer-probe set, showed a sensitivity for detecting IF (at any point) of 93.8%, specificity of 81% and negative predictive value of 94.4%. Finally, the median expression of PTCH1 was found to be significantly higher in remission samples compared to those taken at presentation which supports the hypothesis that the distinct PTCH1 signal detected in diagnostic samples reflects the presence of leukemic cells with a reduced expression of this tumour suppressor gene.
These results are consistent with current hypotheses regarding Hh signaling and highlight its importance in CML pathogenesis. Given the high negative predictive value of high PTCH1 expression for imatinib failure, we propose that its measurement at diagnosis be used to help identify those patients that may require second generation TKIs or other therapies, which may, in future, include Smoothened inhibitors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal