Abstract
Abstract 819
It is widely accepted that inflammation plays an important role in the pathophysiology of Sickle Cell Anemia (SCA). Recently a number of studies indicated that peripheral blood mononuclear cell (PBMC) iron may contribute to inflammation in some diseases In SCA, PBMCs are continuously exposed to high turnover of iron in haptoglobin/hemoglobin complexes and free heme due to severe intravascular hemolysis. Therefore, we hypothesized that PBMC iron flux is marked by altered expression of iron-regulated genes, and in turn associated with the up regulation of genes in inflammatory pathways. To explore this hypothesis we correlated the expression of a predefined set of iron regulated genes to the SCA PBMC transcriptome as a whole.
Steady state patients with SCA were selected as published earlier. (Bereal-Williams et al. Haematologica 2012) Most noteworthy exclusion criteria were recent blood transfusion, liver or kidney dysfunction or a recent acute complication. Affymetrix Human Genome U133 Plus 2.0 arrays were used to measure PBMC mRNA profile. The primary source of free iron in PBMC in SCA is iron released from heme by heme oxygenase-1 (HMOX1), the first committed biochemical step in production of bilirubin. Therefore, we planned to validate the clustering of the study participants by correlating the cluster rank with bilirubin plasma levels. Genes which are published to be differentially expressed upon iron loading or chelation were used to prospectively create an ‘iron-regulated’ gene set (figure). Differentially expressed genes with the filter of a change greater than 40% between the clustering groups and 10% false discovery rate (FDR) were further analyzed with Ingenuity Pathway Analysis (IPA) System.
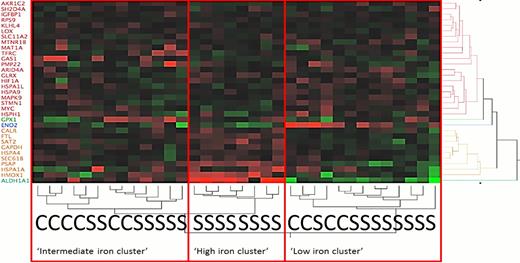
Twenty-five subjects with SCA and 9 healthy subjects were analyzed. Hierarchical clustering using the predefined iron regulated gene list identified 3 groups of subjects with high, low and intermediate expression of iron activated genes (figure 1). As expected, none of the healthy controls was found in the high iron cluster. The cluster grouping was validated by correlation of serum bilirubin levels to the cluster rank (Spearman rho=0.358 p=0.044) and the expression of HMOX1 (Spearmen rho=0.387 p=0.034). In analysis of these three iron regulated gene clusters, we found 98 genes which were consistently and significantly differentially expressed, notably many genes important to crucial inflammatory pathways, especially Toll-like receptor 4 and 7 (TLR-4,7), chemokine receptor CCR1, and interleukin 15. The 98 markers identified ten canonical pathways in Ingenuity Pathway Analysis, most involving inflammation (each p<0.004, Table ).
Expression profiling using a defined set of iron regulated genes identifies co-regulation of genes and pathways related to inflammatory cytokines, signaling cascades and innate immunity pathways. Our human PBMC findings confirm and extend recently presented data from several other groups linking TLR4-mediated heme-iron toxicity to pathological responses in sickle cell mice and in cultured cells. The role of heme-associated iron in sickle cell pathophysiology merits additional investigation.
top ten canonical pathways associated with increased expression of the pre-defined iron regulated gene set. (p<0.004)
| Name . | Differential regulated genes . |
|---|---|
| Role of Pattern Recognition Receptors in Recognition of Bacteria and Viruses | TLR4, NLRP3, TLR7, PRKCH, PRKCA |
| IL-10 Signaling | CCR1, HMOX1, BLVRA, CD14 |
| LPS-stimulated MAPK Signaling | TLR4, CD14, PRKCH, PRKCA |
| Altered T Cell and B Cell Signaling in Rheumatoid Arthritis | TLR4, TNFSF13, IL15(includes EG:16168), TLR7 |
| Communication between Innate and Adaptive Immune Cells | TLR4, TNFSF13, IL15(includes EG:16168), TLR7 |
| Beta-Adrenergic Signaling | GNB4, PRKCH, SLC8A1, PRKCA |
| Phospholipase C Signaling | HMOX1, GNB4, ZAP70, PRKCH, MARCKS, PRKCA |
| Toll-like Receptor Signaling | TLR4, TLR7, CD14 |
| fMLP Signaling in Neutrophils | GNB4, CYBB, PRKCH, PRKCA |
| IL-8 Signaling. | HMOX1, GNB4, CYBB, PRKCH, PRKCA |
| Name . | Differential regulated genes . |
|---|---|
| Role of Pattern Recognition Receptors in Recognition of Bacteria and Viruses | TLR4, NLRP3, TLR7, PRKCH, PRKCA |
| IL-10 Signaling | CCR1, HMOX1, BLVRA, CD14 |
| LPS-stimulated MAPK Signaling | TLR4, CD14, PRKCH, PRKCA |
| Altered T Cell and B Cell Signaling in Rheumatoid Arthritis | TLR4, TNFSF13, IL15(includes EG:16168), TLR7 |
| Communication between Innate and Adaptive Immune Cells | TLR4, TNFSF13, IL15(includes EG:16168), TLR7 |
| Beta-Adrenergic Signaling | GNB4, PRKCH, SLC8A1, PRKCA |
| Phospholipase C Signaling | HMOX1, GNB4, ZAP70, PRKCH, MARCKS, PRKCA |
| Toll-like Receptor Signaling | TLR4, TLR7, CD14 |
| fMLP Signaling in Neutrophils | GNB4, CYBB, PRKCH, PRKCA |
| IL-8 Signaling. | HMOX1, GNB4, CYBB, PRKCH, PRKCA |
Heatmap showing unsupervised two-way hierarchical clustering of the pre-defined set of iron regulated genes on the y-axis and the clustering of study participants on the x-axis. As hypothesized, none of the controls was found to be in the high iron cluster. “S” means SCA patient; “C” means control.
Heatmap showing unsupervised two-way hierarchical clustering of the pre-defined set of iron regulated genes on the y-axis and the clustering of study participants on the x-axis. As hypothesized, none of the controls was found to be in the high iron cluster. “S” means SCA patient; “C” means control.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal