Abstract
Abstract  811
811
High-dose therapy and autologous hematopoietic stem cell transplantation (ASCT) is the standard of care for many patients (pts) with high-risk or relapsed B-cell non-Hodgkin lymphoma (B-NHL). Despite the use of transplant, relapse is likely for most with indolent or mantle cell lymphoma (MCL) as well as for aggressive B-NHL recurring after rituximab (R)-based chemotherapy. Total body irradiation (TBI) has been incorporated into ASCT regimens to overcome chemotherapy resistance and improve outcomes, but studies to date indicate that escalation of TBI is not feasible due to toxicity in non-target organs. We hypothesized that radioimmunotherapy (RIT) could supplant TBI and identified the maximally tolerated dose (MTD) of high-dose RIT using I-131-tositumomab that could be safely combined with high-dose etoposide (VP-16) and cyclophosphamide (CY) prior to ASCT (Press, Blood 2000). We now present the mature data of a large prospective phase II trial at the MTD of high-dose I-131-tositumomab, VP-16, and CY.
Pts were eligible if they were <60 years of age, had MCL in first remission or any relapsed/refractory B-NHL, an ECOG PS of 0–1, acceptable organ function, >2×106 autologous CD34+ peripheral blood stem cells/kg collected, <20 Gy prior radiation to critical organs, and no human-anti-mouse-antibodies. All pts underwent outpatient biodistribution studies using tositumomab (1.7 mg/kg, n= 64 or 485mg flat dose, n= 43) labeled with ∼10mCi I-131 followed by serial quantitative gamma camera imaging to calculate individualized organ-specific absorbed dose estimates. Pts then received therapeutic infusions of I-131-tositumomab to deliver 25Gy to the critical normal organ receiving the highest radiation absorbed dose. When residual radiation was <7mR/hr at 1m (median 10 days) pts received 60mg/kg VP-16 followed 48 hours later by 100mg/kg CY. ASCT occurred when residual radiation was <2mR/hr at 1m. Toxicity was scored on the Bearman transplant scale and CTCAE 2.0. Response followed standard criteria.
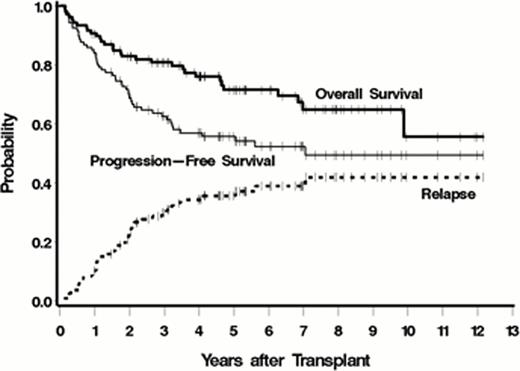
Between June 1999 and April 2011 107 pts were transplanted on this protocol. Baseline characteristics included: median age 52 yrs (range 32–60), stage III/IV = 104 (97%), median number of prior regimens = 3 (range 1–11), prior R = 98 (92%), R refractory = 35 (33%), chemoresistant disease (defined as < a partial response [PR] to the most recent regimen) = 41 (40%), >1 extranodal site = 36 (33%), elevated LDH = 46 (42%). Histological strata: Indolent 43% (45 follicular lymphoma [FL], 1 lymphoplasmacytic lymphoma), MCL = 31%, Aggressive 27% (27 diffuse large B-cell including 12 with transformed disease, 2 Burkitt's). Dose limiting critical normal organs receiving up to 25Gy included lung (71), liver (29), kidney (6), and heart (1) from a median administered I-131 activity of 557 mCi (range 231–1353) resulting in a median whole body dose of 4.4Gy. ASCT occurred an average of 14 days after the I-131-tositumomab infusion with a median of 5.9×106 CD34/kg (range 2–20×106 CD34/kg). An unsupported ANC>500/μL and platelets >20K/μL occurred at a median of day 12 and day 13, respectively. Bearman toxicity of grade III/IV was observed in 6 pts (5.6%) with 3 (3%) non-relapse deaths (all cardiopulmonary toxicity) by day 100. Pulmonary toxicity of ≥ grade 4 CTCAE occurred in 10 (9%) pts. Responses to therapy included: CR/CRu = 87 (81%), PR = 8 (7%), SD = 11 (10%), and PD = 1 (1%). Currently, 76 (71%) pts are alive with 59 (55%) progression-free. The estimated 5-year overall survival (OS), progression-free survival (PFS) and relapse were 72%, 56%, and 36%, respectively (figure, median follow up of 5.2 years). Five-year OS/PFS estimates for the 3 histological groups included indolent (81%/61%), aggressive (69%/51%), and MCL (57%/52%). MCL pts with relapsed/refractory disease had a 5-year OS/PFS of 55%/42%. Multivariable analysis only identified chemoresistant disease as associated with death (HR 3.07 (1.46–6.44, p=.003) and relapse or death (HR 2.12 (1.17–3.86, p=.01). Secondary AML/MDS occurred in 1 patient to-date.
This largest prospective trial of myeloablative RIT demonstrates that I-131-tositumomab delivering 225Gy to critical organs along with high-dose VP-16 and CY prior to ASCT is feasible and effective with toxicities comparable to TBI-based regimens. These data provide the foundation for future approaches incorporating RIT into transplant conditioning regimens for lymphoma.
Gopal:GSK: Research Funding; Spectrum: Research Funding. Off Label Use: high-dose use of I-131-tositumomab. Rajendran:GSK: Research Funding. Pagel:GSK: Research Funding. Maloney:GSK: Research Funding. Press:GSK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal