Abstract
Abstract 808
A subset of patients with Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs) (Polycythemia Vera (PV), Essential Thrombocytosis (ET), and Primary Myelofibrosis (PMF)) subsequently transform to acute myeloid leukemia (AML). Leukemic transformation (LT) after MPN occurs in as many as 23% of PMF patients within 10 years of diagnosis, and in 4–8% of PV and ET patients in the first 18 years after diagnosis. The development of AML after an antecedent MPN is associated with a dismal clinical outcome, and is associated with a poor response to conventional anti-leukemic therapies. Although somatic mutations in the JAK-STAT signaling pathway, including in JAK2 and MPL, occur in the majority of MPN patients, the somatic mutations that drive LT from a pre-existing MPN have not been fully delineated. Recent candidate mutational studies have identified recurrent somatic mutations in a subset of known leukemogenic disease alleles at the time of transformation from MPN to AML, including mutations in TP53, IDH1/2, TET2 and SRSF2 as well as deletions in IKZF1. However, the functional contribution of these specific genetic events to LT has not been delineated, and genetically accurate models of transformation of Philadelphia-chromosome negative MPN to AML have not been reported to date.
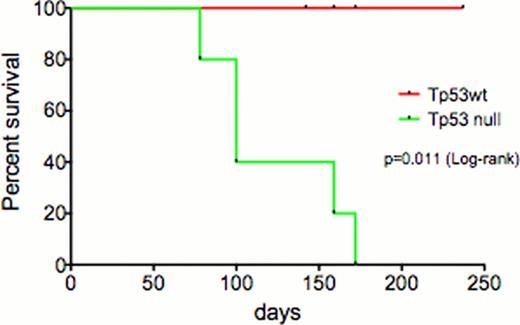
In order to develop a genetically accurate murine model of LT, we have modeled expression of JAK2V617F mutation in combination with TP53 loss in vivo to further our understanding of progression from MPN to AML and to use this preclinical model of LT to test novel therapies. Bone marrow (BM) cells from C57/Bl6 Tp53−/− and littermate control mice were infected with JAK2V617F-IRES-GFP retrovirus, followed by transplantation of transduced cells into lethally irradiated congenic recipients. Of note, transplantation of JAK2V617F/Tp53−/− cells, but not JAK2V617F positive cells was associated with impaired survival; 50% of mice injected with JAK2V617F/Tp53−/− cells died by day 100, whereas all mice injected with JAK2V617F positive cells survived 100 days or longer (p=0.011) (figure 1). Mice injected with JAK2V617F/Tp53−/− cells presented with significant leukocytosis, with a mean WBC of 38.4 in mice engrafted with JAK2V617F/Tp53−/− cells compared with 11.4 in JAK2V617F/Tp53 wildtype mice. At the time of sacrifice, all mice engrafted with JAK2V617F/Tp53−/− cells had increased numbers of blasts in the peripheral blood and bone marrow, as assessed by morphologic evaluation and flow cytometric analysis which noted CD117 expression on leukemic blasts. BM cells from mice engrafted with JAK2V617F/Tp53−/− cells were characterized by increased serial replating (>10 platings), which was not observed in plating studies with JAK2V617F positive cells. In addition, we noted that the disease from JAK2V617F/Tp53−/− cells, but not JAK2V617F positive cells, was transplantable into secondary recipients consistent with increased self-renewal in vivo.
We have begun testing the efficacy of novel therapies in this murine model, using both in vitro assays and in vivo studies in secondary transplantation studies. Treatment with the JAK kinase inhibitors INCB18424 and CYT 387 resulted in dose-dependent inhibition of colony formation in vitro. The combination of INCB18424 and Decitabine (which has demonstrated clinical efficacy in post-MPN-AML) is associated with synergistic inhibitory effects in vitro. Based on these results, we are performing in vivo studies with INCB18424, Decitabine, and INCB18424 + Decitabine, and results from these preclinical therapeutic studies will be presented in detail.
Taken together, our data demonstrate that expression of JAK2V617F plus Tp53 loss, a genoptype commonly seen in patients who transform to AML after MPN, efficiently models LT in vivo. This model can now be utilized to examine the mechanisms of leukemic transformation, including assessment of the leukemic cell of origin in transformed disease. In addition this model can be utilized to test novel therapeutic strategies in a preclinical setting, which can be used to inform clinical trials in this poor-risk hematologic malignancy.
Survival curve of mice transplanted with JAK2V617F in presence and absence of Tp53
Survival curve of mice transplanted with JAK2V617F in presence and absence of Tp53
Verstovsek:Incyte Corporation: Research Funding; Novartis: Research Funding; AstraZeneca: Research Funding; Celgene: Research Funding; SBIO: Research Funding; Lilly Oncology: Research Funding; Bristol-Myers: Research Funding; Geron Corp.: Research Funding; Gilead: Research Funding; YM Biosciences: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Infinity Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal