Abstract
Abstract  804
804
Polycythemia vera (PV) is characterized by increased erythrocyte production resulting in an increased red blood cell mass. White blood cell (WBC) and platelet counts are also elevated and patients have increased thrombotic risk as well as a risk of progression to myelofibrosis (MF) or acute myeloid leukemia (AML). Patients also often have an enlarged spleen and suffer from burdensome symptoms. Dysregulated JAK signaling is linked to PV pathogenesis. Ruxolitinib (RUX) is an oral JAK1/JAK2 inhibitor that is being studied in 2 phase III randomized trials in PV patients (RESPONSE and RELIEF).
To report the long-term efficacy and safety of RUX in PV patients from an open-label, single-arm, phase II study (INCB18424-256; NCT00726232) in patients with PV or essential thrombocythemia (ET).
PV patients were eligible if they were resistant or intolerant to hydroxyurea (HU). Patients were required to have a hematocrit > 45% or 2 phlebotomies in the 24 weeks prior to enrollment, and at least 1 phlebotomy within 12 weeks of enrollment. Response was assessed using a modified version of the European LeukemiaNet (ELN) criteria (Barosi et al. Blood 2009) that at minimum, required hematocrit < 45% without phlebotomy after 4 weeks from first dose. Spleen size was measured by palpation, and blood counts were measured weekly for the first 4 weeks, every 2 weeks for the next 12 weeks, every 4 weeks thereafter for the first year, and then every 12 weeks. At each study visit, patients assessed pruritus, night sweats, and bone pain experienced in the past week on a scale of 0 (absent) to 10 (worst imaginable).
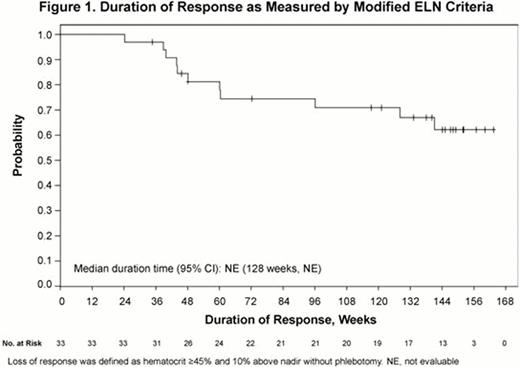
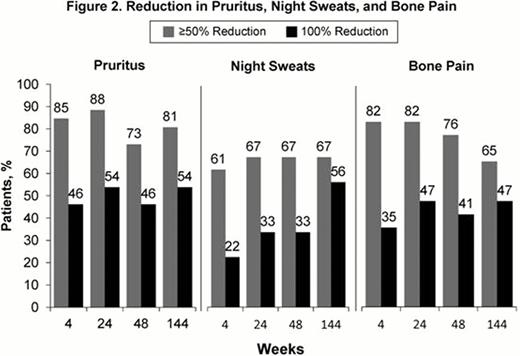
Patients (N=34) were treated with RUX (median dose=21.4 mg/day) for a median duration of 155 weeks (range: 35.3–168 weeks). Overall response (OR) as defined by modified ELN criteria, was achieved in 97% of patients at Week 24. Response was durable with 79% achieving OR at both Weeks 24 and 48. Of these responders, 74% maintained OR at Week 144. The median duration of response has not been reached (Fig 1). Of the 4 patients who were phlebotomized during study, only 1 patient required phlebotomy after Day 15. In patients with WBC count >10 × 109/L at baseline, 84% achieved a WBC ≤10 × 109/L within the first 24 weeks of therapy; 76% achieved this in the 24 weeks preceding Week 144. Similarly, in patients with platelet count >400 × 109/L at baseline, 74% achieved platelet counts ≤400 × 109/L within the first 24 weeks and 61% achieved this in the 24 weeks before Week 144. Among patients with palpable splenomegaly at baseline (n=25), 61% achieved ≥50% reduction in palpable spleen at Weeks 24 and 48; of these, 86% maintained this reduction at Week 144. Additionally, 44% did not have a palpable spleen at Week 24 and 63% did not have a palpable spleen at Week 144. Clinically meaningful improvements in pruritus, night sweats, and bone pain were observed within 4 weeks of initiating therapy and sustained through Week 144 (Fig 2). RUX treatment was associated with reductions from baseline in JAK2V617F allele burden of 8% at Week 48 and 22% at Week 144. Thrombocytopenia and anemia were the most common hematologic adverse events (AEs) and were primarily Grade 1; these events were successfully managed with dose reductions. Grade ≥3 anemia occurred in 3 (9%) patients and Grade ≥3 thrombocytopenia occurred in 3 (9%) patients (n=1 with both). Of these 5 patients, 3 had indications of progression to MF (worsening splenomegaly, leukocytosis, or anemia), and 2 subsequently developed MF on study and were discontinued from study. One patient experienced pulmonary embolism; no other thromboembolic events were reported. No cases of AML were reported. Nonhematologic AEs were predominantly Grade 1 or Grade 2. Twelve patients experienced Grade ≥3 AEs; of these, only 2 patients discontinued from study (renal neoplasm, atrial flutter).
RUX treatment resulted in clinical benefit in PV patients resistant or intolerant to HU by providing durable OR rates (as measured by modified ELN criteria) as well as ameliorating symptoms commonly associated with PV. Treatment was generally well tolerated and the AE profile was similar to that seen with RUX in clinical studies of MF patients. These results suggest that RUX may be an efficacious therapy for PV patients.
Verstovsek:Incyte Corporation: Research Funding. Off Label Use: Ruxolitinib is approved for the treatment of patients with intermediate or high risk myelofibrosis in the United States. Ruxolitinib is not approved for the treatment polycythemia vera. He:Incyte: Employment, Equity Ownership. Contel:Incyte: Employment, Equity Ownership. Mookerjee:Incyte: Employment, Equity Ownership. Kantarjian:Incyte Corporation: grant support for his institution Other. Barbui:Novartis: Honoraria. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal