Abstract
Abstract 730
Carfilzomib is a novel, irreversible proteasome-inhibitor with a significant activity and favourable toxicity profile. Here we evaluated efficacy and safety of the combination carfilzomib-cyclophosphamide-dexamethasone in elderly (≥ 65 years) newly diagnosed MM patients.
The Bryant and Day two-stage design was used to evaluate both efficacy and safety. Patients received oral cyclophosphamide (300 mg/m2 on days 1,8,15), oral dexamethasone (40 mg on days 1, 8, 15, 22) and iv carfilzomib (20 mg/m2 on days 1, 2, and 36 mg/m2 on days 8, 9, 15, 16, cycle 1; 36 mg/m2 on days 1, 2, 8, 9, 15, 16, cycles 2–9) every 28 days for 9 cycles, followed by maintenance with iv carfilzomib (36 mg/m2 on days 1, 2, 15, 16) every 28 days until progression or intolerance.
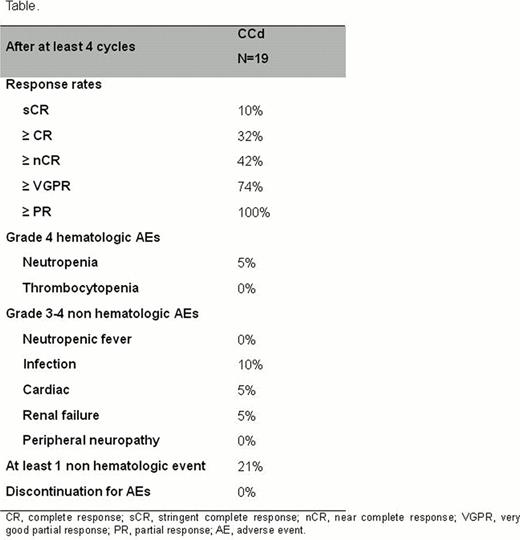
Thirty-four patients were enrolled: median age was 70 years, 21% of patients were older than 75 years, 24% had reduced creatinine clearance (<50 mL/min), 46% had ISS stage III, and 28% had unfavorable FISH [t(4;14) or t (14;16) or del17p]. Nineteen patients were evaluated for response and for safety after at least 4 cycles (table). All patients achieved at least partial response (PR), 74% at least very good partial response (VGPR), 42% at least complete response (CR)/ near-CR, including 10% stringent-CR. Responses were rapid with the median time to PR of 1 month and the median time to CR of 2 months. After a median follow-up of 7.5 months no patient has progressed or died.
Grade (G) 4 hematologic toxicities included neutropenia (1 patient, 5%). At least one G3-4 non-hematologic event was reported in 4 patients (21%): pneumonia and bronchitis (2 patients, 10%), atrial fibrillation (1 patient, 5%) and renal failure (1 patient, 5%). Only 1 patient (5%) developed G1 peripheral neuropathy. No patient discontinued treatment and 4 patients (21%) required carfilzomib dose reductions due to adverse events (G4 neutropenia, G3 pneumonia, G3 atrial fibrillation and G3 renal failure).
Carfilzomib-cyclophosphamide-dexamethasone showed encouraging activity in patients with newly diagnosed MM. The combination was well tolerated with a frequency of at least one G3-4 non-hematologic adverse event of 21%. Results will be updated at the meeting.
Palumbo:Onyx: Honoraria. Bringhen:Onyx: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal