Abstract
Abstract 726
Circulating Tumor Cells (CTCs) are present in the vast majority of MM patients, but the biologic basis for the movement of CTCs from the bone marrow (BM) to the PB circulation is not clear. Are all BM myelomatous plasma cells (mPCs) capable to egress into PB, or only a specific sub-clone? Do CTCs have stem cell-like features and are enriched by clonogenic cells? Notably, hematopoietic stem cells (HSCs) also circulate in PB, under a specific circadian rhythm orchestrated by the central clock promoting the regeneration of the stem cell niche in other sites of the BM during the resting period; does circadian rhythms also affect CTCs?
In the present study we investigated the phenotypic, functional and circadian characteristics of CTCs from symptomatic MM patients (at diagnosis or relapse), comparing them with patient' paired BM mPCs. The immunophenotypic characterization (n=13) was performed using 8 color multiparameter flow cytometry (MFC). CTCs and BM mPCs were sorted by MFC according to (patient specific) aberrant phenotypes, and clonogenic growth (n=6) was evaluated by platting the same number of cells in co-culture with the human BM stromal cell line (hTERT) at 10:1. Colonies consisting of more than 40 cells were scored at day 14. Proliferation (n=10) was measured using MFC with the DRAQ5 dye. The circadian rhythm of CTCs and HSCs in the PB of MM patients at relapse (n=5) was investigated by MFC starting at 16:00pm and quantifying both cell populations every 4h up to 12:00am next day (when patients' initiated treatment). Plasma was simultaneously collected and the levels of SDF-1 were measured using a quantitative ELISA assay.
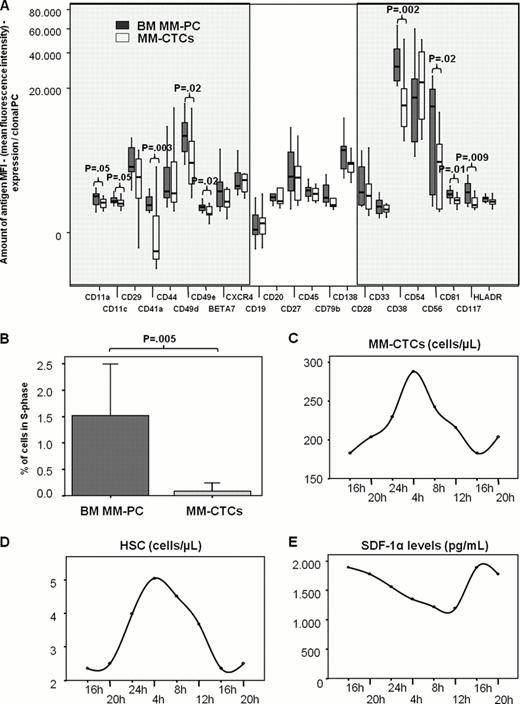
Among 20 antigens analyzed, CTCs differed (P≤.05) from paired BM mPCs by a down-regulation of the mean fluorescence intensity of the integrins CD11a, CD11c, CD41a, CD49d and CD49e (all these molecules known as mediators of cell adhesion and motility) (Figure 1A). Similarly, expression of the adhesion molecules CD38 and CD56 was significantly decreased in CTCs, and so was the stem-cell factor receptor CD117. CD81, a tetraspanin involved in B-cell activation and proliferation was also down-regulated in CTCs. In turn, no differences were noted (P>.05) for maturation-related markers such as CD19, CD20, CD27, CD45, CD79b, and CD138, as well as CXCR4.
We then investigated the proliferation index of CTCs vs. BM mPCs (Figure 1B). Our results show a marked reduction of PCs in S-phase in the fraction of CTCs as compared to their counterpart in the BM (mean values of 0.07% vs. 1.5%; P=.005). In turn, colony formation assays showed that when co-cultured with human BM stromal cells, CTCs have a 3-fold enhanced clonogenic capacity as compared to paired BM mPCs.
Finally, we investigated whether the number of CTCs followed a specific circadian rhythm similarly to that reported for HSCs. Strikingly, we founded that CTCs exhibited circadian fluctuations (Figure 1C), peaking at 4:00am (288 cells/μL) and reaching a low point at 16:00pm (183 cells/μL). This pattern was also found for normal HSCs (Figure 1D), with mean numbers peaking at 4:00am (5.0 cells/μL) and reaching a nadir at 16:00pm (2.4 cells/μL). Conversely, median SDF1 levels fluctuated in anti-phase with CTCs and HSCs, peaking at 16:00pm (1892 pg/mL) and dropping at 12:00am (1198 pg/mL).
In summary, our results show that CTCs represent a sub-clone of BM mPCs characterized by a unique profile of integrin and adhesion molecules, but without sign of being at a different phenotypic maturation stage. CTCs are mostly quiescent, but show enhanced clonogenic potential as compared to BM mPCs when co-cultured with stromal cells. Similarly to HSCs, CTCs show a circadian rhythm triggered by rhythmic expression of SDF1 which may indicate that as the former, CTCs egress to PB during patients' resting period to colonize other sites in the BM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal