Abstract
Abstract 4803
Very little literature is available on aggressive transformation of indolent T-cell large granular lymphocytic leukaemia (LGL). We present the first report of such a case occurring in the context of chemotherapy.
A 50 year old female with a ten year history of T-cell LGL presented with a systemic deterioration, fevers, and abdominal discomfort. At the time of diagnosis LGL morphology was typical, with normal cytogenetics, and an immunophenotype of CD3+, 8+, 16+, 56−, 57low. In the years following the original diagnosis, she suffered with recurrent infections due to persistent neutropenia and had received several courses of treatment with variable efficacy, including ciclosporin, methotrexate, splenic radiotherapy, and intermittent G-CSF.
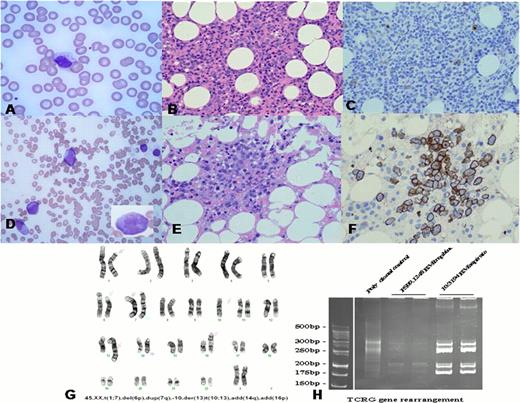
At the time of deterioration, the spleen was grossly enlarged at 30 cm. FBC showed Hb 9.8 g/dL, WBC 8.2 × 109/L, lymphocytes 7.3 × 109/L, neutrophils 0.56 × 109/L, and platelets 70 × 109/L. The blood film showed large granular lymphocytes with typical morphology (Fig. 1A). A bone marrow trephine revealed an interstitial infiltrate of small T-cells (Fig. 1B) which were CD30− (Fig. 1C) and flow cytometry showed these cells to be CD3+, CD7+, CD8+, CD16−, CD56−, CD57low.
The patient had a transient response with prednisolone and was commenced on fludarabine and cyclophosphamide (FC). FC was well tolerated, and after two cycles symptoms had improved, no organomegaly was palpable, and the cytopenias had resolved. After the third cycle, the patient was admitted with neutropenic sepsis. FBC showed Hb 12.3 g/dL, WBC 0.5 × 109/L, neutrophils 0.18 × 109/L, and platelets 49 × 109/L. The blood film revealed frequent large, immature cells with nucleoli and inconspicuous granules (Fig. 1D, inset). These cells were abundant in the bone marrow (Fig. 1E), were CD30+ (Fig. 1F) and 10 of 10 cells examined had a complex karyotype (Fig. 1G). 3 of 10 cells had this karyotype prior to chemotherapy, indicating that clonal progression had occurred prior to treatment, although morphologically blastic cells were absent at that point. PCR analysis of TCRG revealed identical band patterns from the time of the original diagnosis, the time of progression 10 years later, and the point of final deterioration 4 months subsequently (Fig. 1H). Despite aggressive resuscitation, the patient passed away.
The data indicate that a subclone of this patient's longstanding T-LGL had undergone cytogenetic transformation whilst initially retaining typical morphology. Significant clinical deterioration at the time prompted treatment with FC. After four months of therapy the morphology became blastic. It is difficult to speculate on the possible role of chemotherapy in the development of blast crisis. TCRG analysis confirmed the clonal relationship between the cells present at the initial diagnosis and those that underwent blastic transformation.
Although aggressive forms of LGL leukaemia are well described, they are usually of NK cell origin and do not present with an indolent phase.1 True de novo aggressive T-cell LGL has also been documented, although without a clinically indolent phase.3 Ohno et al first described transformation of indolent CD3- CD16+ LGL in 1989, documenting trisomy of chromosome 8 in both the chronic and aggressive phases.3 Molecular evidence of aggressive transformation of T-cell LGL has been documented previously.4
This is the first report of aggressive transformation of T-LGL in the context of chemotherapy. In patients with indolent T-cell LGL who deteriorate clinically, the possibility of aggressive transformation should therefore be actively investigated, and cytotoxic chemotherapy administered with caution.
No relevant conflicts of interest to declare.
References
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal