Abstract
Abstract 4726
The thrombopoietin receptor agonists (TPO-RA), romiplostim and eltrombopag, are presently indicated for the treatment of certain patient groups with immune thrombocytopenia purpura. In a clinical study with romiplostim in patients with low-/intermediate-1 risk myelodysplastic syndromes (MDS), cases of transient increases in blast cell counts were observed and cases of MDS disease progression to acute myeloid leukemia (AML) were reported.
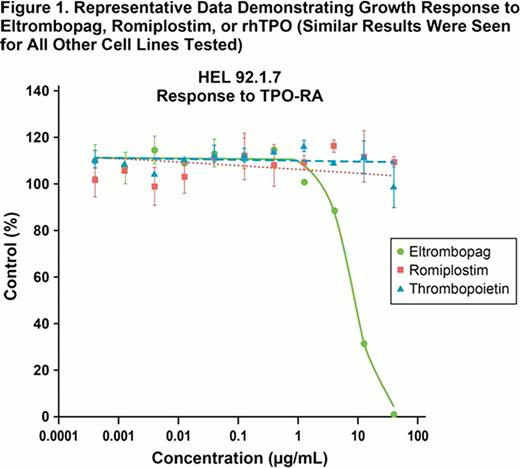
In the present study, we evaluated the impact of romiplostim, eltrombopag, and recombinant human thrombopoietin (TPO) on the proliferation of 5 human AML and 1 TPO-dependent megakaryoblastic cell line. The cell lines evaluated include the TPO-dependent cell line, N2C TPO; the TPO-R positive AML lines, HEL92.1.7 and OCI-AML-3; and the TPO-R negative AML cell lines, HL60, THP-1, and NOMO-1. All cells were exposed to 11-point dose response curves of the 3 agents at concentrations sufficient to generate a full stimulatory response in the N2C TPO cell line. Cells were exposed to concentrations of romiplostim and eltrombopag that met or exceeded the reported Cmax achieved for each agent in high-dose clinical trials and were 3- (eltrombopag) to 30-fold (romiplostim) above trough levels from the same clinical trials.
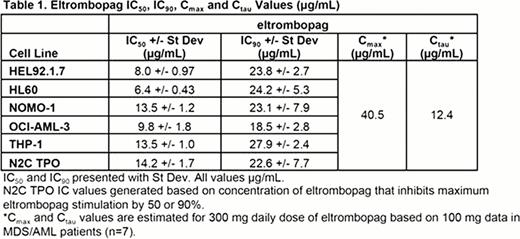
Neither romiplostim nor TPO treatment resulted in detectable stimulation or inhibition of leukemia cell growth at concentrations up to 10 μg/mL. Treatment with eltrombopag up to 40 μg/mL caused inhibition of all AML cell lines with mean IC50 values ranging from 6.4 to 13.5 μg/mL. These IC50 values reflect concentrations that are 3- to 6-fold below the Cmax of a 300 qd dose of eltrombopag (40.5 μg/mL) and at concentrations as low as 2-fold below Ctau levels (12.4 μg/mL). Cmax exceeded the IC90 for these AML cell lines, which ranged from 18.5 to 27.9 μg/mL. No stimulation of AML growth was evident through the range of the eltrombopag dose response curve on any of the cell lines evaluated. The results of this study confirm earlier in vitro studies (Will 2009, Erickson-Miller 2010) showing inhibitory effects of eltrombopag on leukemic cell lines and support clinical studies to evaluate a potential anti-leukemic effect of higher doses of eltrombopag in patients with AML.
Rusnak:GlaxoSmithKline: Consultancy, Equity Ownership, Patents & Royalties. Off Label Use: Eltrombopag is an oral TPO agonist indicated for chronic ITP being studied in MDS/AML. Rudolph:GlaxoSmithKline: Consultancy, Equity Ownership. Erickson-Miller:GlaxoSmithKline: Employment, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal