Abstract
Abstract 4581
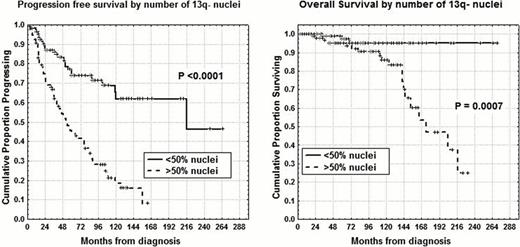
Cytogenetic aberrations are considered major prognostic indicators for predicting the survival of chronic lymphocytic leukemia (CLL) patients (Dohner et al, 2000) and interphase fluorescent in situ hybridization (I-FISH) with specific probes is generally used to detect the most frequent abnormalities. Moreover, deletion 13q14.3 on FISH analysis which is the most common cytogenetic abnormality in CLL is a favorable prognostic biomarker when detected as a sole abnormality, even if a higher percentage of 13q- nuclei was found to be associated with significantly shorter time to treatment (P<0.001), (van Dike et al, 2010; Dal Bo et al, 2011). Therefore, the primary endpoints of our research were: 1) to determine progression free survival (PFS) and overall survival (OS) on the basis of percentages of 13q- nuclei and 2) to confirm 13q14 number of deleted cells as an independent prognostic factor. We investigated 503 pts, median age 65 years (range 33–89), 291 males and 212 females. With regard to modified Rai stages at diagnosis, 163 had a low stage, 320 an intermediate stage and 20 a high stage. Probes for chromosome 13q (LSI-D13S319), 11q (LSI-ATM), 17p (LSI-p53) and chromosome 12 (CEP12) were used on nuclei collected at diagnosis. One hundred fifty-three patients (30.4%) exhibit a normal karyotype, 203 pts (40.4%) showed an isolated 13q-, 63 pts (12.5%) presented trisomy 12, 49 pts (9.7%) 11q deletion, 26 (5.2%) 17p deletion and 9 (1.8%) other chromosome abnormalities. Clearly, patients with intermediate/poor cytogenetic abnormalities (trisomy 12, del11q-, del17p-) showed significant shorter PFS and OS (7% vs 36% at 14 years and 45% vs 77% at 14 years, P<0.0001) in comparison with normal or del13q- pts. Maximally selected log-rank statistics identified the 50% of nuclei bearing del13S319 as the most appropriate cut-off value capable of separating del13q- cases into two subgroups with different PFS and OS distributions. In fact, pts with isolated 13q- in <50% of nuclei (110 pts) showed a longer PFS and OS (62% vs 16% at 12 years, P<0.0001 and 95% vs 47% at 16 years, P=0.0007, Figure) compared to those with ≥50% of nuclei (93 pts). Noteworthy, 64 (69%) of 93 of 13q- >50% pts had received chemotherapy at the time of analysis, whereas only 27 (25%) of 110 of 13q- <50% pts had been treated (P <0.0001). There was no significant clinical difference between heterozygous and homozygous 13q- patients as well as between 13q- cases with RB1 deletion (delRB1) and 13q- without delRB1. There was a significant correlation between number of 13q deleted nuclei and number of B-lymphocytes/microliter (P<0.0001) at diagnosis as well as between 13q- nuclei percentages and lymphocyte doubling time (P=0.001), meaning that 13q- nuclei represent both the amount of disease and the proliferation rate in CLL. Only slight significant correlations were found between 13q- percentages and CD38 (P=0.04) or ZAP-70 (P=0.01) or IgHV status (P=0.03), whereas 36 of 54 (67%) CD69+ pts had del13q- >50% (P=0.0003). In the context of del13q- subset, multivariate analysis of PFS confirmed the percentage of nuclei (P=0.00007) together with IgHV status (P=0.003) and ZAP-70 (P=0.0002) as an independent prognosticator. With regard to OS, percentage of nuclei (P=0.02) together with age (P=0.009) and IgHV status (P=0.03) was again confirmed as an independent variable. Therefore, the percentage of nuclei exhibiting 13q- at diagnosis has to be considered an important predictor of the clinical outcome and the clinical implications of an isolated 13q deletion in CLL appear more complex and important than originally considerated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal