Abstract
Abstract 454
Allogeneic hematopoietic stem cell transplantation (HSCT) was initially developed as a rescue from therapy-related bone marrow failure following high dose chemo/radiation therapy to rescue from therapy-related bone marrow failure, the emphasis has shifted towards allogeneic HSCT as a strategy to facilitate graft-versus-tumor (GVT) activity. Strategies to suppress graft-versus-host diesase (GVHD) are often associated with broader suppression of the immune system leading to immune deficiency and compromised GVT activity.
The aim of this study was to modulate T cell responses in the context of GVHD by targeting the NF-kappaB family member c-Rel, a transcription factor that upon antigen receptor triggering regulates lymphocyte survival and proliferation. In T cells, the main target gene of c-Rel is interleukin 2 (IL-2), a cytokine required for normal T cell proliferative and differentiative responses. The effect of c-Rel deficiency on T cell differentiation and function has been investigated previously demonstrating that c-Rel deficient T cells show reduced Th1, Treg and Th17 but normal Th2 responses. Inhibition of c-Rel is therefore expected to impair T cell activation, differentiation, and function. Using methods to inhibit the c-Rel pathway we developed a novel strategy to minimize the severity of GVHD while leaving GVT activity intact.
We investigated the role of c-Rel during GVHD in MHC mismatched as well as MHC matched, minor antigen mismatched (more clinically relevant) murine HSCT models. c-Rel−/− T cells caused significantly less GVHD than normal T cells, as determined by survival (p<0.001), clinical GVHD score (p<0.01), weight loss, and histopathology of GVHD target organs (p<0.001 liver; p<0.001 gut, p<0.05 skin). Time course analyses revealed that 3 days post transplant, proliferation and activation of c-Rel−/− T cells was impaired (p<0.001 CFSElow/high ratio; p<0.01 CD25+ donor cells) and pSTAT5 expression was decreased (p<0.05), resulting in less expansion of donor derived effector T cells by day 7 after transplant (p<0.001). Interestingly, serum levels of IL-2 were increased on day 7, likely secondary to decreased pSTAT5-mediated negative feed back on IL-2 secretion, and we observed increased numbers of donor derived Tregs with increased CD25 expression in recipients of c-Rel−/− T cells (p<0.05), suggesting that the increased IL-2 levels primarily benefited the expansion of Tregs. Levels of Th2 cytokines (IL-4, IL-5 and IL-13) were increased and GATA-3 expression was up-regulated in recipients of c-Rel−/− T cells (p<0.05), implicating a shift toward Th2 responses.
We next evaluated if c-Rel−/− T cells were able to mediate antitumor activity. We challenged allogeneic HSCT recipients with either a liquid tumor (A20 lymphoma) or a solid tumor (RENCA-renal cell carcinoma) and found that GVT activity in c-Rel−/− T cells recipients was intact in the absence of GVHD, resulting in significantly improved survival compared to recipients of wildtype T cells (p<0.001 A20; p<0.01 RENCA). Using a syngeneic GVT model with EL4 (T cell lymphoma) we could demonstrate strong anti-tumor activity of c-Rel−/− T cells in the absence of T cell alloactivation, reinforcing the notion of separation of GVHD from GVT activity through inhibition of c-Rel signaling.
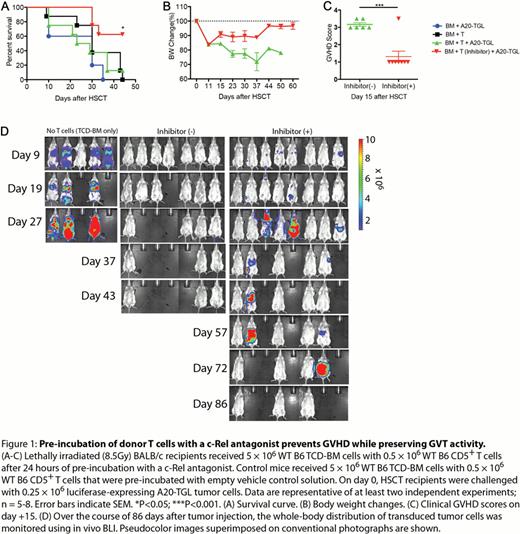
The potential for clinical translation of our approach is highlighted by a series of experiments testing the efficacy of a recently developed Pyrimidinetrione-based small molecule c-Rel inhibitor compound. We found that incubation of T cells with this compound resulted in efficient downregulation of c-Rel expression as well as IL-2 expression in vitro. When we administered T cells that were pre-incubated with this c-Rel antagonizing small molecule, we were able to reproduce our above described findings regarding the effect of c-Rel deficient T cells on GVHD (Figure 1). Importantly, normal T cells pre-incubated with a c-Rel antagonist mediated intact GVT activity, indicating that they are viable and functional T cells. This strongly suggests that the diminished capacity of these cells to induce GVHD is due to the c-Rel inhibitory mechanism of the small molecule compound and not due to a non-specific cytotoxic effect of the in vitro manipulation procedure.
Taken together, our findings identify c-Rel as a promising target for the development of a clinical strategy to prevent or ameliorate GVHD while preserving GVT activity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal