Abstract
Abstract 446
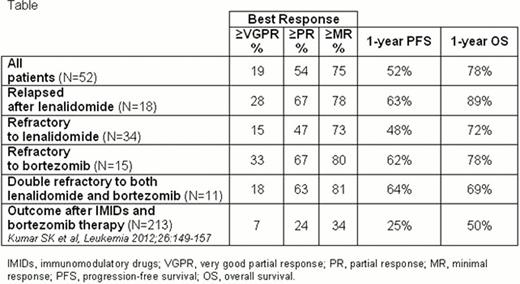
The outcome of myeloma (MM) patients who are no longer responding to thalidomide, lenalidomide or bortezomib is poor, with a median event-free survival of 5 months and median overall survival (OS) of 9 months (Kumar SK et al, Leukemia 2012). The newer immunomodulatory drug pomalidomide, has shown significant activity in these clinical conditions.
We assessed dosing, efficacy and safety of pomalidomide-cyclophosphamide-prednisone (PCP) in MM patients relapsed/refractory to lenalidomide.
Pomalidomide was administered in doses ranging from 1 to 2.5 mg/day on days 1–28, cyclophosphamide at 50 mg every other day on days 1–28 and prednisone at 50 mg every other day on days 1–28 for 6 cycles, followed by maintenance therapy with pomalidomide-prednisone. Thromboprophylaxis with aspirin 100 mg/day or low-molecular weight heparin was recommended at physician's discretion.
The maximum tolerated dose (MTD) of pomalidomide was defined as 2.5 mg/day. Fifty-two patients were enrolled at the MTD and evaluated after completing at least 1 PCP cycle. Median age was 69 years (range 41–83). The median time from diagnosis to enrolment was 55 months (range 15–203). Best responses to PCP included 6% of complete response (CR), 19% of at least very good partial response (VGPR), 54% of at least partial response (PR) and 75% of at least minimal response (MR). Time to PR was rapid (median 1.8 months). After a median follow-up of 11 months (range 1–18), 1-year progression-free survival (PFS) and OS rates were 52% and 78%, respectively. PFS was not significantly different in patients with high-risk cytogenetic compared with patients with standard-risk disease and in patients younger or older than 75 years.
Toxicities were primarily hematologic and included grade 4 neutropenia (13%) and thrombocytopenia (4%). At least grade 3 non-hematologic toxicities included infections (8%), rash (6%) and neurologic (6%). Thromboembolism occurred in 1 patient. Four patients discontinued treatment for toxicity (2 infections, 1 neurologic and 1 hepatic toxicity).
PCP induced high response rates and prolonged PFS after prior exposure to lenalidomide and bortezomib (Table), without adding significant toxicity. PCP could be considered a valuable salvage option for pre-treated MM patients.
Palumbo:Celgene: Consultancy, Honoraria. Larocca:Celgene: Honoraria. Sciacca:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Guglielmelli:Celgene: Honoraria. Giuliani:Celgene: Research Funding. Boccadoro:Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal