Abstract
Abstract 4427
Very rarely, Philadelphia positive (Ph+) chronic myeloid leukemia (CML) presents with isolated thrombocythemia (CML-T) with no or only slight elevation of neutrophil counts. Due to their rarity, such patients have not been studied in detail, especially whether they constitute a separate disease entity, where the initiating leukemic hit originates from a more lineage-committed progenitor cell type than the one affected in classical CML. To evaluate this, we studied a 68-year-old woman who during a follow-up for follicular lymphoma presented with a sudden thrombocythemia of 2062×109/L, a basophil count of 0.94×109/L but no increase in neutrophil count (3.9×109/L). A bone marrow (BM) biopsy revealed slight to moderate hyperplasia with an increased number of small, hypolobulated megakaryocytes, but no increase in the myelopoietic compartment. Apart from the Ph1 positivity, no molecular aberrations were seen by cytogenetic analysis, array CGH, or extensive molecular testing; noteworthy, the JAK2 V617F mutation was absent. The patient was treated with three different Tyrosine Kinase Inhibitors (TKI's), all of which had to be terminated after a total of 26 weeks of therapy due to severe side effects (dyspnea, acrocyanosis of the fingers and fatigue). At present, she is on pegylated interferon alpha-2a, which is well tolerated.
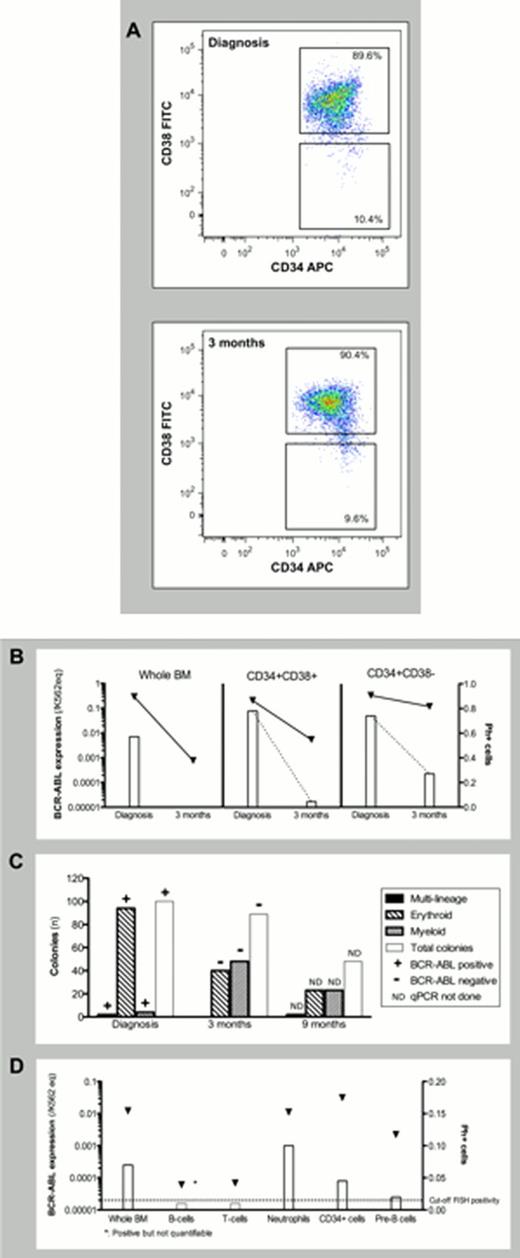
In a longitudinal study of the stem cell biology in this patient we centered on three BM aspirates obtained at diagnosis as well as 3 and 9 months following diagnosis. Subsets of progenitor- and mature lineage committed cells were FACS-purified and analyzed for the presence of leukemic cells by FISH and qPCR technique and additionally subjected to growth in a 14-day methylcellulose assay. At diagnosis, the CD34+ compartment consisted of 10% immature (CD34+CD38-) and 90% mature (CD34+CD38+) progenitors, both of which were enriched for Ph+ cells (74% and 78%, respectively) (Figure 1A-B). Moreover, the colony-forming cells (CFC's) from sorted CD34+ cells were BCR-ABL+ with the erythroid lineage (BFU-E; 94%) dominating and only 4% myeloid lineage (CFU-G, CFU-M, and CFU-GM) and 2% multi-lineage (CFU-GEMM). By qPCR analysis of picked colonies we, moreover, observed that all types of colonies were BCR-ABL+ (Figure 1C). After three months of TKI therapy the patient responded with a 2.6 log decrease in BCR-ABL expression in BM mononuclear cells, the BCR-ABL+ CFC's disappeared and the distribution of erythroid and myeloid colonies normalized. However, specific analysis of the progenitor compartment showed reductions of only 0.5 log in CD34+CD38- cells and 1.6 log in CD34+CD38+ cells. At 9 months after the diagnosis, after pausing TKI treatment for 3 months, BCR-ABL expression in peripheral blood resurged and further analysis identified Ph+ cells by FISH in CD66+ neutrophils (10%) and CD19+CD10+ B-cell precursors (2%), and by expression of BCR-ABL in CD19+ B-cells (positive in one of three triplicate wells) and CD3+ T-cells (0.000068 K562 equivalents) (Figure 1D).
These data show, despite the highly unusual presentation with isolated thrombocythemia, that the origin of this form of CML is very similar to classical CML with pan-lineage involvement and a resistance of progenitor cells to TKI therapy. The selective expansion of megakaryocytic lineage cells in this form of CML, however, remains enigmatic and will require further insight into the mechanics of differentiation in the malignant clone at the level of common myeloid progenitors or earlier.
Delineation of stem cell biology in a CML patient presenting with isolated thrombocythemia. (A) FACS sorting of CD34+CD38− and CD34+CD38+ cells at diagnosis and 3 months after. (B) Initial treatment response in whole BM and FACS sorted CD34+CD38+ and CD34+CD38− cells. (C) Lineage involvement of the CML clone 9 months after diagnosis (after 3 months of pausing TKI treatment). (D) Growth of FACS sorted CD34+ cells in CFC-assay at diagnosis and 3 and 9 months after.  : BCR-ABL expression measured by qPCR, and normalized to K562 equivalents (K562eq).
: BCR-ABL expression measured by qPCR, and normalized to K562 equivalents (K562eq).  : Fraction of Ph+ cells evaluated by FISH.
: Fraction of Ph+ cells evaluated by FISH.
Delineation of stem cell biology in a CML patient presenting with isolated thrombocythemia. (A) FACS sorting of CD34+CD38− and CD34+CD38+ cells at diagnosis and 3 months after. (B) Initial treatment response in whole BM and FACS sorted CD34+CD38+ and CD34+CD38− cells. (C) Lineage involvement of the CML clone 9 months after diagnosis (after 3 months of pausing TKI treatment). (D) Growth of FACS sorted CD34+ cells in CFC-assay at diagnosis and 3 and 9 months after.  : BCR-ABL expression measured by qPCR, and normalized to K562 equivalents (K562eq).
: BCR-ABL expression measured by qPCR, and normalized to K562 equivalents (K562eq).  : Fraction of Ph+ cells evaluated by FISH.
: Fraction of Ph+ cells evaluated by FISH.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal