Abstract
Abstract 4428
The Fas death receptor (CD95/TNFSFR6) conveys death and non-death signals through binding to its cognate ligand, FasL (CD95L)1,2. Fas is expressed constitutively in CD34+ cells of patients with chronic myeloid leukemia3. In order to explore the implication of Fas in CML patient's response, we aimed at analyzing expression and function of Fas in the imatinib-sensitive human Bcr-abl+ Fas+ AR 230S cell line, and in its imatinib-resistant counterpart AR 230R.
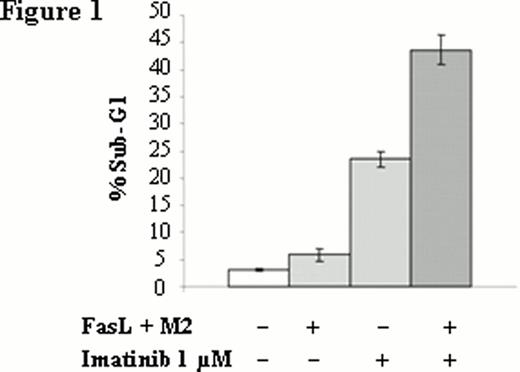
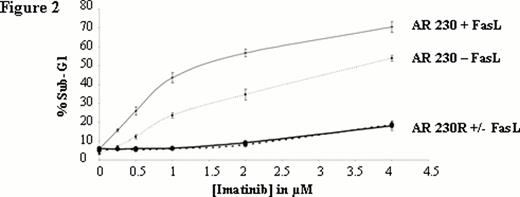
We analysed the Fas-mediated apoptosis of AR230S and showed that not only imatinib (Figure 1) but also nilotinib and dasatinib (data not shown) potentiate Fas-L mediated apoptosis. This potentiation is dose dependent (Figure 2). Interestingly, there is no potentiation of TKI-mediated apoptosis by FasL in the imatinib-resistant counterpart AR 230R which is Fas negative. The Fas knockdown with a lentivirus-expressing a FAS-shRNA in AR 230S was associated with a disappearance of potentiation effect, whereas the up regulation of Fas expression in AR230 S cell line using lentivirus-expressing human Fas (hFas) induced an emphasized potentiation effect confirming the pivotal role of Fas in the potentiation of TKI-mediated apoptosis.
Notably, IFN-α is able to enhance expression of Fas on CD34+ cells from CML patients3. We thus explored the action of IFN-α on the Fas potentiation of TKI-mediated apoptosis. We observed that preincubation with IFN-α increased potentiation in a dose-dependant manner (Data not shown).
In conclusion, our preliminary results demonstrate the pivotal role of Fas in the potentiation of TKI-mediated apoptosis and highlight the role of IFN-α in the design of CML treatments.
Percentage of FasL-mediated apoptosis in AR230 cell line. Apoptosis induced by FasL in presence or in absence of imatinib was analyzed by propidium iodide staining of cellular DNA content and shown as percentage of sub-G1 to the whole population.
Percentage of FasL-mediated apoptosis in AR230 cell line. Apoptosis induced by FasL in presence or in absence of imatinib was analyzed by propidium iodide staining of cellular DNA content and shown as percentage of sub-G1 to the whole population.
Dose-dependentof FasL mediated apoptosis in absence or in combination with imatinib. Apoptosis induced by FasL in presence or in absence of imatinib analyzed by propidium iodide staining of cellular DNA content and shown as percentage of sub-G1 to the whole population. Each point represents the mean ± S.E.M. of 3 experiments.
Dose-dependentof FasL mediated apoptosis in absence or in combination with imatinib. Apoptosis induced by FasL in presence or in absence of imatinib analyzed by propidium iodide staining of cellular DNA content and shown as percentage of sub-G1 to the whole population. Each point represents the mean ± S.E.M. of 3 experiments.
Legros:Novartis: Research Funding, Speakers Bureau; BMS: Speakers Bureau; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal