Abstract
Abstract 4351
Wilms‘ tumor protein-1 (WT1) is one of the most investigated tumor-associated antigens (TAA) in hematological malignancies. CD8 T-cell responses against several WT1-derived peptides have been characterized and are known to contribute to disease control after allogeneic hematopoietic stem cell transplantation (HSCT). Also the identification of human leukocyte antigen (HLA) class II-restricted CD4 T-cell epitopes from WT1 is a challenging task of T-cell-based cancer immunotherapy to improve the effectiveness of WT1 peptide vaccination.
We found a highly immunogenic WT1 peptide composed of only 9 amino acids having the ability to induce IFN-γ secretion in CD4 T-cells in an HLA DR-restricted manner. This finding is of great interest as it was generally accepted that HLA class II binding peptides are composed of at least 12 amino acids being recognized by CD4 T-cells, whereas HLA class I binding peptides are composed of 8–11 amino acids being recognized by CD8 T-cells (Wang et al Mol. Immunol. 2002). However, both HLA class I and class II molecules bind to primary and secondary peptide anchor motifs covering the central 9–10 amino acids. Thus, considering this common structural basis for peptide binding there is a possibility that the WT1 9-mer peptide binds to HLA class II molecules, and induces CD4 T-cell responses.
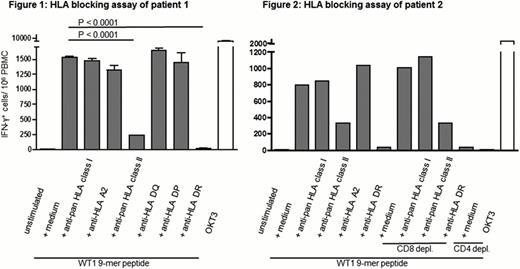
IFN-γ induction in response to several WT1 9-mer peptides was screened in 24 HLA-A*02:01 positive patients with acute myeloid leukemia or myelodysplastic syndrome after allogeneic HSCT. Responses to one WT1 9-mer peptide were exclusively detected in CD3+CD4+ T-cells of 2 patients after allogeneic HSCT, but not in CD3+CD4+ T-cells of their corresponding HSC donors. CD4+ T-cell responses to this WT1 9-mer peptide exhibited high levels of functional avidity, as IFN-γ induction was detected after stimulation with 100 ng peptide per mL. Peptide-induced IFN-γ production was confirmed with IFN-γ ELISPOT assays and the HLA restriction of the T-cell response was determined by HLA blocking antibodies. The reaction was significantly blocked by anti-pan HLA class II antibody (85 % reduction), but neither by pan-HLA class I nor by anti-HLA A2 antibody. To identify the subtype of HLA class II molecule, blocking assays with antibodies against HLA-DP, HLA-DR and HLA-DQ were performed. IFN-γ induction was completely abrogated by anti-HLA-DR antibody (99 % reduction) (fig 1, p value of unpaired student‘s t-test <0.0001 for the medium control vs anti-pan HLA class II antibody or anti-HLA-DR antibody, respectively). To test whether IFN-γ was exclusively induced in CD4 T cells, CD4 or CD8 T-cells were depleted from PBMC. Whereas CD8 T-cell depletion did not affect IFN-γ induction, CD4 T-cell depletion completely abrogated the WT1 9-mer peptide induced response (fig 2). CD4 T-cells responding to the WT1 9-mer peptide were indicated to be functional cytotoxic T-cells with an effector CD4 T-cell phenotype. Longitudinal analyses demonstrated the persistence and functionality of WT1 9-mer specific CD4 T-cells in PBMC of patients even at day 1368 after allogeneic HSCT.
These data indicate for the first time that a TAA-derived 9-mer peptide can induce HLA class II-restricted CD4 T-cell responses. Vaccination with the characterized WT1 9-mer peptide can enhance the induction and maintenance of not only CD4 but also indirect CD8 T-cell responses. Considering that CD4 T-cells play an important role in tumor rejection, the possibility that other TAA-derived 9-mer peptides having the potential to induce CD4 T-cell responses should be explored in other settings of tumor immunology as well to improve vaccination strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal