Abstract
Abstract 4269
High-dose chemotherapy with autologous stem cell rescue (HDT-ASCT) remains the standard of care for patients with chemosensitive, relapsed diffuse large B-cell lymphoma (DLBCL). Because of concern for unacceptable toxicity and therefore inferior outcomes, geriatric patients (age >= 70) with relapsed DLBCL are typically not considered candidates for HDT-ASCT in clinical practice and have largely been excluded from clinical trials examining the role of HDT-ASCT with curative intention. There is a paucity of data in the transplant literature describing outcomes (with long-term follow up) of geriatric DLBCL patients undergoing HDT-ASCT in the relapsed-refractory setting.
Utilizing Kaplan-Meier survival and Cox regression analyses, we conducted a two-center (John Theurer Cancer Center and Weill-Cornell Cancer Center) retrospective cohort study to evaluate the outcome of geriatric patients (age >= 70) with chemosensitive, relapsed-refractory DLBCL who underwent HDT-ASCT between 1994 and 2011. The primary study endpoint was overall survival (OS) assessed by chart review and SSDI database. As a secondary endpoint, we examined survival outcomes of geriatric patients with relapsed-refractory DLBCL who underwent HDT-ASCT in the rituximab era (transplant performed after year 2000).
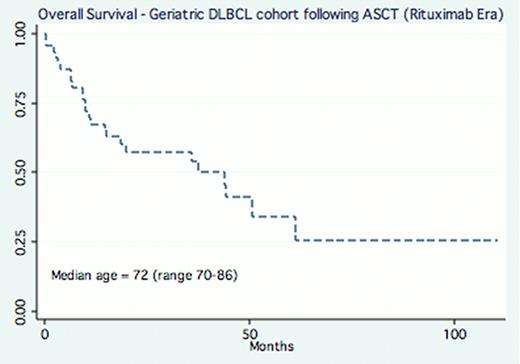
75 geriatric patients with lymphoma (DLBCL 71%, MCL front-line consolidation in CR 11%, HL 5%, FCL 4%, PTCL 4%) were identified in our combined transplant outcomes database of which 52 underwent HDT-ASCT (n=40 JTCC, n=12 Cornell) for relapsed-refractory DLBCL representing 30 deaths and 1522 total months at risk (median follow up 18.4 months). Baseline characteristics included: median age at time of salvage and ASCT was 72, (range 70–86), ECOG PS 1 (range 0–2), ethnicity (90% caucasian), sex (65% male), hematopoietic cell transplant-co-morbidity (HCT CI) index score (median=1), conditioning regimen (BEAM 49%, BEAC 33%, 16% CBV, 2% Cy-TBI). With a median follow up of 18.4 months, the median OS for the entire cohort was 35.7 months with a 100 day mortality of 8.6% (95% CI 7.4–9.3%). The median OS for DLBCL patients who underwent HDT-ASCT in the rituximab era was 43 months (Figure). In univariate analysis, age (continuous variable, p=.15), patient sex (p=.9), HCT CI score (continuous variable, p=.4) were not associated with inferior survival.
HDT-ASCT in a geriatric patient population is feasible with similar outcome as younger pts as shown by the following:
ASCT was associated in our series with comparable OS to younger patients with relapsed lymphoma.
For patients with relapsed/refractory DLBCL treated with rituximab in the front line setting, outcomes are similar to published data (CORAL study, EFS at 3 yr=21%) in younger patients.
Overall given the incidence of NHL in elderly (reported increase >500% in > 80y over last 10 years), a larger number of pts might have to be considered to salvage and ASCT.
Additional prospective studies should explore optimization of HDT-ASCT in the geriatric setting including upcoming novel strategies from combination with new biologicals, to drug antibody conjugates as well as maintenance post ASCT to help continue improve the outcome in this population.
Mato:Celgene: Speakers Bureau; Millennium: Speakers Bureau; Seattle Genetics: Speakers Bureau; Genentech: Speakers Bureau. Goy:Milennium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; J & J: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Feldman:Merck: Speakers Bureau; Seattle Genetics: Speakers Bureau; Celgene: Speakers Bureau; Allos: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal