Abstract
Abstract 4155
Cord blood (CB) transplant (CBT) can be curative for patients with high-risk hematologic malignancies. However, patients of older age and/or with significant co-morbidities do not tolerate CBT with high-dose myeloablative conditioning. Non-myeloablative (NMA) conditioning can reduce transplant-related mortality (TRM) and extend transplant access to older or infirm patients, but it is limited by the risks of graft rejection in patients without extensive prior chemotherapy and relapse. While the addition of anti-thymocyte globulin (ATG) may reduce rejection, it increases the risk of viral infections, including Epstein-Barr virus lymphoproliferative disease, and may also increase relapse risk.
We investigated the safety and efficacy of an ATG-free regimen of intermediate intensity prior to double-unit CBT in 30 patients with acute leukemias and myelodysplasia. Units were 4–6/6 HLA-A, B antigen, DRB1 allele matched to the patient. The conditioning regimen included cyclophosphamide 50 mg/kg (day -6), fludarabine 30 mg/m2/day × 5 (days -6 to -2), thiotepa 5 mg/kg/day × 2 (days -5 and -4), total body irradiation 200 cGy × 2 (days -2 and -1), and cyclosporine-A/mycophenolate mofetil immunosuppression. The indication for this regimen was one or more risk factors for TRM including age > 50 years, extensive prior therapy, and/or significant co-morbidities. The hematopoietic cell transplant co-morbidity index (HCT-CI) score of Sorror was retrospectively assigned.
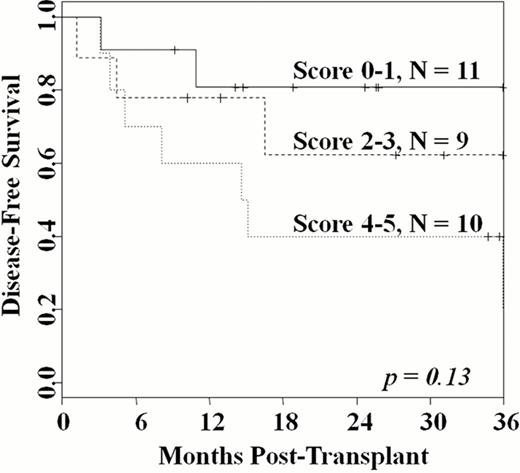
The median age was 56 years (range 18–69). All but one patient had high-risk disease. Twenty-one had AML (16 CR1, 5 CR2) with all CR1 patients having high-risk features, including high-risk cytogenetics (n = 3), FLT-3 ITD mutation (n = 5), therapy-related disease or prior MDS (n = 6), and/or > 3 consecutive induction chemotherapies (n = 2). Five had ALL (4 CR1, 1 CR3); the 4 in CR1 had BCR/ABL mutations (n = 3) or prior refractory CNS disease (n = 1). Four patients had MDS with 3 having an IPSS score > 2. The median HCT-CI score was 2.5 (range 1–5). Median infused TNC doses were 2.6 (larger unit) and 1.9 (smaller unit) × 107/kg, respectively. Ninety-seven percent of patients engrafted (95%CI: 87–100) at a median of 26 days (range 13–43). The median day 21 total donor bone marrow chimerism was 100% (range 71–100). All surviving patients were 100% donor by day 100, and sustained hematopoiesis has been mediated by a single unit in all but one patient. The cumulative incidence of platelet recovery > 20 × 109/L by day 180 was 93% (95%CI: 83–100), and occurred at a median of 46 days (range 30–79). Day 180 TRM and 2-year relapse incidences were 20% and 11%, respectively. With a median 26.5 months (range 9–53) follow-up of survivors, the 2-year overall survival and disease-free survival (DFS) are both 60% (95%CI: 44–82). There was a hierarchy in 2-year DFS according to the Sorror HCT-CI score (Figure): the 11 patients (median age 55 years) with a score of 1 had a DFS of 82%. This compared with a 2-year DFS of 62% in the 9 patients (median age 51 years) with a score of 2–3, and 40% in the 11 patients (median age 58 years) with a score of 4–5 (p = 0.13).
This reduced intensity regimen combined with double-unit CBT reliably facilitates sustained donor engraftment without ATG. This regimen is associated with less toxicity than high-dose myeloablative conditioning. While other approaches are needed in patients with high comorbidity scores, this regimen is highly effective in older patients who are otherwise reasonably fit, as evidenced by the 82% 2-year DFS in patients with a median age of 55 years. Given the relatively low risk of relapse, it also represents a promising alternative to high-dose conditioning in younger patients.
Giralt:Onyx: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal