Abstract
Abstract 3858
Standard therapy for high-risk MDS consists of one of two FDA-approved demethylating agents, azacitidine or decitabine. AML patients considered unfit for intensive chemotherapy are also frequently treated with one of these agents, both of which have activity in AML and an acceptable toxicity profile. These agents are not considered curative, and responses are of variable duration. When azacitidine or decitabine is not effective, or is no longer effective, few options are available. In view of differences in pharmacological properties between these two agents, it is not uncommon to switch demethylating agents after failure of initial demethylating agent therapy. To date, only two small studies have addressed the outcome of this intervention, with conflicting results. In a study of 14 MDS patients treated with decitabine following failure of, or progression on, azacitidine, 4 patients (28%) responded (Borthakur et al, Leuk Lymph 2008), while a subgroup analysis of 10 evaluable patients treated with decitabine following azacitidine failure demonstrated no complete or partial responses (Prebet et al, J Clin Oncol 2011). Here we report outcomes in a retrospective analysis of 22 MDS and AML patients who received decitabine after azacitidine.
Charts of 22 MDS or AML patients who were evaluated at the University of Maryland Greenebaum Cancer Center between January 1, 2008 and March 31, 2012 and who received decitabine after azacitidine were reviewed.
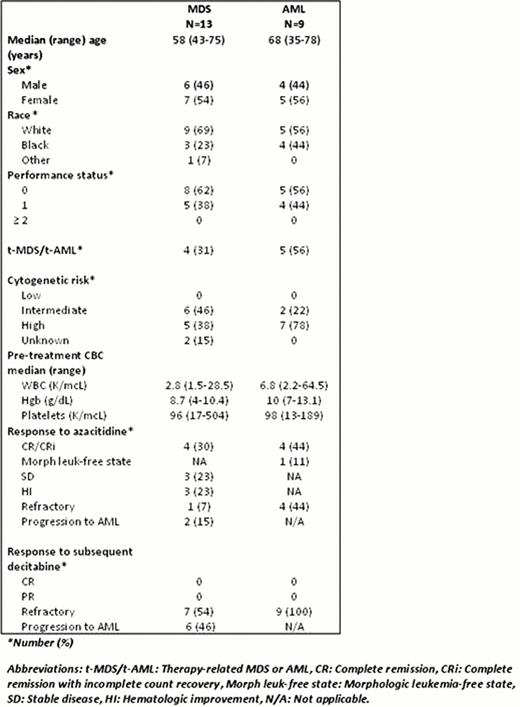
Patient and disease characteristics for 13 MDS patients and 9 AML patients are shown in Table 1. All patients were initially treated with azacitidine before being switched to decitabine following lack of response to initial therapy or disease progression after initial response. Five of 9 AML patients received azacitidine as frontline therapy, while four received it after other induction chemotherapy regimens.
Disease responses following azacitidine 75mg/m2 for 5–7 days in 28-day cycles were variable as shown in Table 1. The median number of courses of azacitidine to disease response for MDS and AML patients who responded was 5 (range, 2–10), median disease-free survival (DFS) was 6.1 months (range, 2.3–16), and median number of courses of azacitidine received was 9 (range, 1 to 17). All 22 MDS and AML patients were refractory to, or progressed after, subsequent decitabine therapy after receiving a median of 2 courses (range, 1–6).
Our study population represents the largest to date and the first in which AML disease responses to decitabine after failure of azacitidine have been studied. All MDS and AML patients were refractory to decitabine therapy following initial treatment with azacitidine. Thus this approach should generally not be employed. New therapies are needed for patients for whom demethylating agent therapy is, or becomes, ineffective, and these patients should be enrolled on clinical trials whenever possible.
Baer:Novartis, Inc.: Research Funding; Celgene, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal