Abstract
Abstract 3736
Despite the 3–5h half-life of the tyrosine kinase inhibitor (TKI) dasatinib (Das), patients with chronic myeloid leukemia (CML) receiving a once daily dose of 100mg achieved similar cytogenetic and molecular responses as patients on 50mg twice a day. We previously reported that high dose short-term dasatinib exposure (100nM for 30 min) induces cell death and results in complete inhibition of Bcr-Abl, which is reversed within 2h of drug washout. Recently, it has been suggested that retention of low Das levels, following transient exposure to high drug doses, is responsible for the observed in vitro effect on cell survival. Here we investigate the mechanism of cell death in the setting of incomplete Bcr-Abl kinase inhibition.
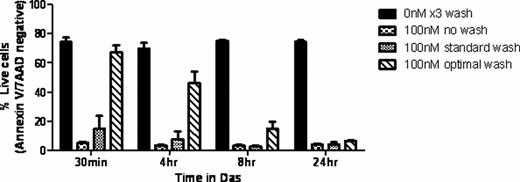
Das treatment was investigated in KU812 and Meg01 BCR-ABL-positive CML cell lines using Annexin V/7-AAD staining and cell proliferation assays (at 72h, n=3). Continuous low dose (LD) Das (1nM) induced cell death (83% and 88%) which was accompanied by an immediate loss of pSTAT5 and pErk, and reduction of Bcl-XL, Mcl-1 and Bcl-2 expression. Initial inhibition of pBcr-Abl was poor, however, it was fully inhibited by 48 hours, suggesting that loss of survival signalling through pSTAT5 and pErk prior to inhibition of pBcr-Abl promotes cells death in this setting. Transient exposure to higher dose Das (100nM) for 30min followed by drug washout (STD wash, 3 × 10ml PBS, at 37°C) and re-culture to 72h in the absence of drug, induced cell death (90.2% and 91%) similar to continuous LD culture. Inhibition of pSTAT5 and pErk also preceded inhibition of p-Bcr-Abl, suggesting the presence of residual Das after washout.
Adoption of an optimal drug washout approach, where cells equilibrate for 1h between washes following 30min exposure to 100nM Das, resulted in a significant reduction in cell death (13.6% and 5.9%). Following this optimal washout (OPT wash), no inhibition of pBcr-Abl was observed and transient inhibition of pErk and pSTAT5 was reversed by 4h. Association of cell survival with recovery of pSTAT5 and pErk suggests that inhibition of these signals by low level Das, whether in continuous LD or the STD wash setting, is critical to Das-induced cell death.
STAT5 and Erk activation occur through various pathways, therefore we interrogated pathways known to facilitate escape from cell death. We and others have previous shown that inhibition of the JAK/STAT pathway prevents cytokine survival signalling, and that inhibition of TKI-induced autophagy induces cell death. We therefore assessed cell survival and proliferation in the presence of a JAK1/2 inhibitor (INCB-018424, Active Biochem) or pSTAT5 inhibitor (pimozide, Sigma) to target cytokine signalling pathways and chloroquine, an autophagy inhibitor, in the presence and absence of 100nM Das (OPT wash) to assess their potential to sustain the otherwise reversible effect of short-term Das priming. Interestingly, neither inhibition of JAK1/2 nor autophagy enhanced cell death, however pimozide plus Das eradicated live cells after OPT wash (1.6%) while pimozide alone had minimal impact on cell survival (89%) emphasising the key role of STAT5 in CML survival signalling.
To translate these observations to a more clinically relevant setting, Das exposures of 100nM were prolonged to 4 and 8 h (analogous to the in vivo half life and activity of the drug) prior to OPT wash. Both conditions resulted in significant cell death in KU812 and Meg01 cells comparable to continuous treatments (Figure 1), which was associated with pBcr-Abl inhibition contrary to the 30 min drug treatment.
Effect of various treatments on KU812 cells
This study demonstrates that a low level of Das (either 1nM continuous dose or residual Das following 100nM transient exposure) induces apoptosis in CML cells despite minimal Bcr-Abl inhibition. Cell death induced by low level Das may be tightly coupled to inhibition of pSTAT5 and pErk achievable with only partial kinase inhibition. Removal of residual Das using an optimal washout reverses apoptosis, but can be overcome by longer, clinically relevant Das exposure or concomitant inhibition of pSTAT5. Our findings enable a better understanding of the potential clinical effectiveness of low dose Das treatment and will help establish the critical CML signalling components which may be targeted in combination therapeutic approaches.
Nievergall:CSL: Research Funding. Hiwase:CSL: Research Funding. White:Novartis Oncology: Honoraria, Research Funding; BMS: Research Funding; CSL: Research Funding. Hughes:Novartis Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal