Abstract
Abstract  3552
3552
There is a substantial data to suggest that immune response to acute promyelocytic leukemia (APL) contributes significantly towards achieving durable remission. The impact of arsenic trioxide (ATO) on immune reconstitution, when used to treat APL, has not been studied. Single agent ATO is able to induce complete molecular remission in practically all patients who complete an induction and consolidation course. However, while ATO is well tolerated approximately 20% of newly diagnosed cases will relapse with such a regimen. We undertook a prospective study to evaluate the pattern of immune reconstitution in patients with newly diagnosed APL treated at our center with a single agent ATO regimen.
Peripheral blood samples were obtained from patients before treatment, on day 15 after starting ATO, post induction, pre consolidation, maintenance cycle 2 (6 months from diagnosis) and maintenance cycle 6 (10 months from diagnosis) for flow cytometry analysis. Briefly cells were labeled using a panel of monoclonal antibodies to CD3, CD4, CD8, CD56, CD16, CD19, CD45RO, CD45RA directly conjugated with FITC, PE, PerCP or APC followed by red cell lysis and cells were then washed and analyzed using FACS Calibur. Cells were gated using forward versus side scatter dot-plots. Lymphocyte subset percentages were calculated from the lymphocyte gate and absolute lymphocyte subset counts were calculated for the analysis.
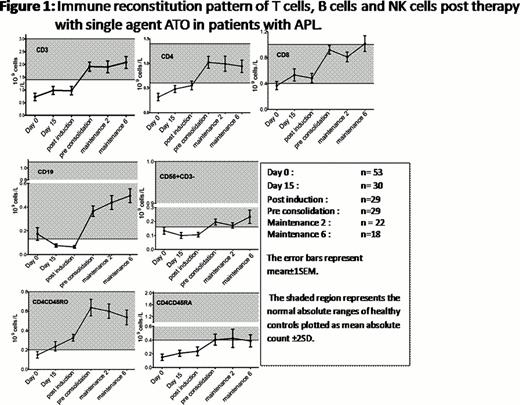
Analysis of lymphocyte subset reconstitution patterns have shown that all the subsets were below the normal range at diagnosis. Following treatment there was differential pattern of immune reconstitution in different lymphocyte subsets. The earliest recovery to normal range was seen in the CD8 subset (Figure 1) while the mean CD4 subset reached the normal range only at 3 months from diagnosis. A normal CD4 to CD8 ratio had not been reached even at the last follow up in this study (10 months from diagnosis). There was a significant delay in the immune reconstitution of NK cells (CD56+CD3-) and naïve T cells (CD4+CD45RA+). The pattern of immune reconstitution of some of the lymphocyte subsets that were evaluated is summarized in Figure 1.
The number of relapses in this cohort was too few to make any association between relapse and the pattern of immune reconstitution. However, we had previously reported that RT-PCR positivity at the end of induction was a significant independent risk factor for subsequent relapses with this regimen. Among the 29 cases available for evaluation at the time of completion of induction and documentation of hematological remission 14 (48.2%) were RT-PCR negative. At this time point there was a significantly higher number of NK-T cells (CD56+CD3+) in those that were RT-PCR negative versus those that were positive (6.1±4.5 × 107/Lt Vs. 2.9±4.1 × 107/Lt; P=0.040). There was also a trend to lower CD3 and a lower CD56br/CD16dim subset in the cases that were RT-PCR positive at the end of induction.
This study demonstrates that there is significant heterogeneity in the pattern of immune reconstitution in different lymphocyte subsets post treatment of APL with ATO. Modulation of immune recovery and response could potentially improve leukemia clearance and maintenance of remission.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal