Abstract
Abstract 3524
Although Notch signaling contributes to T cell leukemogenesis, the role of Notch in human AML is unclear. We and others have found that activation of Notch signaling inhibits AML growth and survival, e.g. a tumor suppressor like effect. However it is not known what the consequences of activating or inhibiting Notch signaling are in human AML in vivo.
To determine whether Notch signaling would have growth inhibiting effects in vivo, we stably-transduced ML1 human AML cells with the constitutively-active forms of Notch1 and Notch2 (ICN1, ICN2), the common Notch target gene Hairy/Enhancer of Split 1 (HES1) or the pan-Notch inhibitor dominant-negative Mastermind-like (dnMAML) and performed in vivo competitive proliferation assays. Briefly, following transduction, each vector type were sorted to 50% GFP+ (containing the gene of interest) and GFP- (untransduced control cells). Groups of NSG mice were injected with these 50:50 mixtures of ML1 cells, and peripheral blood levels of GFP- and GFP+ cells were measured with flow cytometry for anti-human CD45 and GFP.
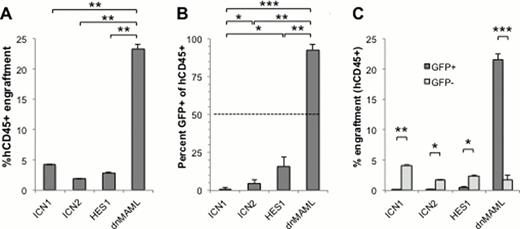
Peripheral blood engraftment of human CD45+ ML1 cells by week 5 was similar (2–5%) for ICN1, ICN2 and HES1 injected mice, but was significantly higher (23%) in dnMAML injected mice (Panel A). Similar to in vitro competitive proliferation assays, ICN1, ICN2 and HES1 all led to decreased relative numbers of GFP%+ cells, with 1%, 4% and 16% respectively (Panel B). Importantly, dnMAML had little effect on AML proliferation in vitro, however led to dramatic increases in GFP% as well as early morbidity and mortality due to increased leukemia burden, with 93% GFP+ (Panel B), demonstrating a selective advantage for dnMAML-expressing ML1 in vivo. When GFP+ (transduced) ML1 peripheral engraftment was directly compared to GFP- (parental CD45+) engraftment, the GFP- control cells had similar engraftment rates (2–5%) across groups of mice, while the GFP+ engraftment rates were significantly lower in ICN1, ICN2, and HES1 groups, but significantly higher in the dnMAML group (Panel C), demonstrating enhanced engraftment/proliferation in dnMAML-expressing cells.
This suggests a previously unreported concept, namely that endogenous Notch ligands can inhibit human AML growth in vivo. This data supports the hypothesis that Notch behaves as a tumor suppressor in AML, and suggests the potential use of Notch agonists in human AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal