Abstract
Abstract 3300
Hemostasis is an important physiologic process that requires the aggregation of platelets at distinct sites of vascular injury to promote clot formation and prevent blood loss. Platelet response to soluble agonists and shear stress has been studied extensively, but little is known of how microenvironmental geometry affects platelet function. As platelets must quickly adhere to, aggregate, and initiate coagulation only at the affected areas, spatial cues must at some level regulate this process. This aspect of spatial regulation has been investigated under static conditions by our group and others (Kita et al., 2011; Van de Walle et al., 2012). Understanding this aspect of platelet function is vital for better understanding the process of hemostasis and pathophysiological conditions such as thrombosis. Here, we directly examine how spatial cues affect platelet aggregation and physiology under variable shear conditions by flowing heparinized whole blood over micropatterned collagen in a microfluidic channel. This system allows us to assess platelet aggregate morphology under different geometric constraints and shear rates, as well as evaluate platelet physiology at the single cell level by measuring calcium signaling using fluorogenic dyes.
A microfluidic channel was bonded to a glass coverslip stamped with FITC-conjugated Type I collagen using a novel technique combining microcontact printing and the stamp-stick bonding technique (Satyanarayana et al., 2005). Before flowing, each chamber was incubated with 1% bovine serum albumin (BSA) blocking solution for 1.5 hours. Whole heparinized blood was then flowed through the chamber at shear rates of 100, 1000, and 10000 s−1. Platelets were labeled with Fura Red, and time lapse confocal imaging was performed for 10 minutes to monitor the aggregation of platelets at the start of flow. The flow chambers were then flushed with Tyrode's buffer with 0.1% BSA using the same experimental shear rates until the chamber was cleared of red blood cells. Image analysis was conducted using ImageJ (to calculate the percentage of platelet coverage on the collagen stamps at different shear rates.
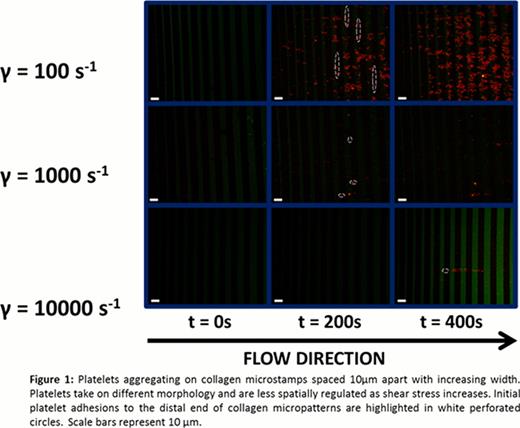
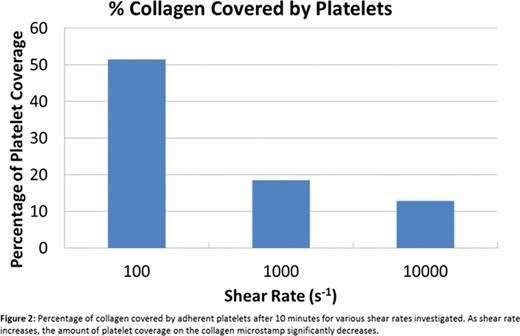
Platelets initially adhere to the distal edge of the collagen micropatterns for all shear rates (Fig. 1), indicating that platelets may require a priming region before forming a stable adhesion. As shear rate increased, platelet coverage of the collagen stamps decreased. However, aggregates also grew without conforming to the geometric constraints imposed by the collagen micropatterns more frequently at those higher shear rates (Fig. 1). Though platelet tethers generally aligned in the direction of the flow, increased tether lengths could be seen when platelets were exposed to higher shear, which may explain why platelets were able to span larger gaps and aggregate in a less spatially constrained manner at high shear rates. Image analysis shows that 51.5% of the collagen was covered by platelet aggregates for a shear rate of 100 s−1 with some platelets forming tethers to span gaps (Fig. 2). When the shear rate was increased to 1000 s−1, platelet coverage of the collagen microstamp drastically dropped to 18.5% (Fig. 2). At a pathophysiological shear rate of 10000 s−1, the percentage of collagen covered by platelets dropped further to 12.8% and adopted a linear shape, although a large portion of the aggregate can be seen spanning gaps between the collagen microstamp (Fig. 1 and 2).
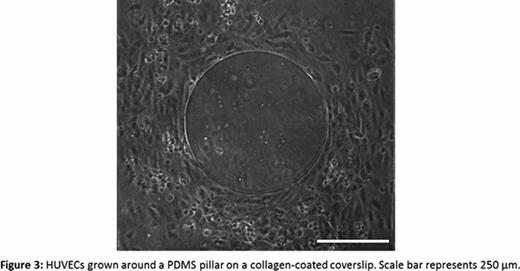
Ours is the first reported study of the spatial regulation of platelet aggregation under variable shear in a microfluidic channel. We have found that platelets are more spatially regulated under low shear conditions compared to high shear, which has implications for thrombosis and other clotting disorders. Future studies will incorporate the simultaneous use of ratiometric fluorgenic calcium signaling dyes to investigate the role of spatial regulation in Ca2+ signaling. Finally, we have developed a method to culture endothelial cells around a collagen micropattern to study this spatial regulation of platelet function under more physiological conditions (Fig. 3).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal