Abstract
Abstract 3281
Regulatory γδT cells (γδTregs) are capable of suppressing T-cell activation and proliferation (Rita et al Journal of immunology 2009). Our previous studies demonstrated the immunosuppressive features of γδTregs and further supported the use of γδTregs as a promising therapeutic strategy for the adoptive immunotherapy of allograft rejection, post-transplantation graft-versus-host disease (GVHD) and autoimmune diseases (Hu et al ASH abstract 2011). To this end, large-scale, efficient expansion of γδTregs represents a fundamentally important prerequisite for their clinical application. Here we explored the efficacy of decitabine (a DNA hypomethylating agent) for the generation of functional γδTregs and its underlying mechanisms.
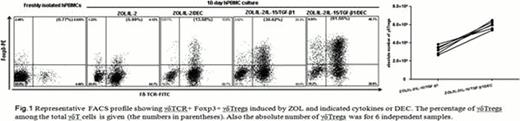
Human peripheral blood mononuclear cells (PBMCs) were cultured with interleukin (IL)-2, IL-15, TGF-β1 and zoledronic acid (ZOL). Decitabine was added to aliquots of PBMCs. Frequency and absolute number of γδTregs were investigated by flow cytometry (FACS) and trypan blue live-cell counts after cultivation. Our results showed a higher frequency (61.9±10.3% versus 38.4±7.8%, p<0.01) and absolute number of γδTregs [(3.1±0.1)×106 versus (5.9±0.2)×106, p<0.01] were seen in cells treated with decitabine plus ZOL/IL-2/IL-15/TGF-β1 (referred to as decitabine-induced γδTregs below) than in cell without decitabine induction (referred to as common γδTregs) as shown in Fig. 1. These data demonstrated that decitabine and ZOL/cytokines synergized to induce stable γδTregs via improved Foxp3 expression.
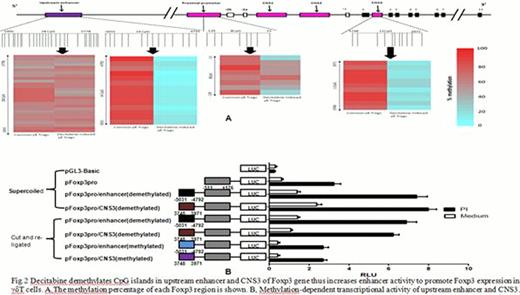
Next we analyzed the underlying mechanisms of decitabine on γδTreg induction. Genomic DNA was isolated from γδTregs sorted by FACS. The Foxp3 gene methyaltion status of upstream enhancer CpG islands from −5995 to −5778 and from −5031 to −4792, proximal promoter CpG island from -139 to -15 and non-coding DNA sequence 3 (CNS3) CpG island from 3748 to 3971 was analyzed by bisulfite sequencing. We found decitabine-induced γδTregs displayed efficient CpG demethylation of the upstream enhancer from −5031 to −4792, and of CNS3 from 3748 to 3971, but no differences were detected in the other CpG islands investigated in contrast to common γδTregs (Fig. 2A). We subsequently investigated if DNA demethylation of CpG islands could influence upstream enhancer or CNS3 activity to promote Foxp3 transcription. The upstream enhancer from −5031 to −4792 and CNS3 from 3748 to 3971 were methylated using SssI (CpG) methylase, and methylated or unmethylated sequences were inserted into demethylated pFoxP3Pro, respectively. Vectors were added to γδT cells and electroporated using the T-023 program of the Nucleofector. Dual fluorescence-based transient reporter assays showed an obvious reduction in luciferase activity after methylation of both CpG islands, but not with the demethylated CpG islands (Fig. 2B), indicating that demethylation of the upstream enhancer and CNS3 can promote Foxp3 expression via increased enhancer activity.
Transcription factors (TF) play important roles in gene expression. To test whether decitabine enhanced specific TFs expression to influence γδTreg induction, we performed TF CHIP assays in FACS-sorted common γδTregs versus decitabine-induced γδTregs. The expression levels of 245 TFs were evaluated and the result showed that NF-κb expression in decitabine-induced γδTregs was up-regulated 50 times in contrast to that in common γδTregs. NF-κb up-regulation was further comfirmed by western blot analysis from 6 different healthy volunteers.
Finally, We evaluated the immunosuppressive function of the enriched γδTregs on Carboxyfluorescein Diacetate (CFSE)-labeled hPBMC proliferation and activation stimulated with anti-CD3/anti-CD28 monoclonal antibodies for 5 days in vitro. As shown in Fig. 3, both common γδTregs and decitabine-induced γδTregs exerted dose-dependent suppression of proliferation, but decitabine-induced γδTregs had a greater suppressive effect than common γδTregs.
In conclusion, these data demonstrate for the first time that decitabine facilitates the generation of functional γδTregs via foxp3 gene demethylation and NF-κb up-regulation. Futhermore, our results lay the foundation for large-scale availability of induced γδTregs for potential clinical applications, and reveal novel targets for regulating Foxp3 expression in γδT cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal