Abstract
Abstract 3194
ACE-536 is a recombinant fusion protein consisting of a modified form of the extracellular domain of the human activin receptor type IIB (ActRIIB) linked to the human IgG1 Fc domain. ACE-536 acts as a ligand trap for members of the TGF-β superfamily involved in erythropoiesis. ACE-536 promotes late-stage erythrocyte precursor cell differentiation by inhibiting specific TGF-β family ligands. Studies of ACE-536 in wild type animals across several species demonstrated an erythroid response that was rapid in onset, robust, and sustained. ACE-536 was also shown to significantly improve hematologic parameters and correct ineffective erythropoiesis in mouse models of myelodysplastic syndromes (MDS) and β-thalassemia. This study is the first human clinical trial of ACE-536.
This was a single-center, randomized, double-blind, placebo-controlled, multiple ascending dose study to evaluate the safety, tolerability, PK, and PD effects of ACE-536 in healthy, postmenopausal women. Screening and baseline hemoglobin values were to be between 11.0 and 14.5 g/dL. Sequential cohorts of 8 subjects each were randomized to receive either ACE-536 (n=6) or placebo (n=2) administered as SC injections on Day 1 and Day 15. ACE-536 dose levels tested (prior to halting dose escalation per protocol due to positive hemoglobin response) were 0.0625, 0.125 and 0.25 mg/kg. The study included pre-specified individual stopping rules for the Day 15 dose for increases in hemoglobin, changes in blood pressure, or ≥ grade 3 adverse events. From Day 1 through Day 57, subjects were assessed for safety by monitoring adverse events, clinical laboratory tests, ECG, vital signs and physical examination. Longer-term follow up visits occurred on Days 71 and 127.
A total of 32 subjects were enrolled. The mean (SD) age was 59.4 (5.8) yr (range: 49–71 yr). All subjects in the first cohort (0.0625 mg/kg or placebo) received both doses of ACE-536; all subjects in the second cohort (0.125 mg/kg or placebo) received only one dose of ACE-536 due to study interruption. This dose level was repeated for the third cohort in which subjects received 2 doses; 1 subject in that cohort did not receive the second dose due to elevated blood pressure. Four subjects in the fourth cohort (0.25 mg/kg or placebo) did not receive the second dose due to hemoglobin increase ≥ 1.0 g/dL and one additional subject did not receive the second dose due to an AE of papular rash.
There were no serious adverse events or study discontinuations reported. The majority of AEs were considered mild in severity. No clinically meaningful abnormalities in laboratory measures, vital signs, physical exam, or ECG were observed, and no anti-drug antibodies were detected. The mean serum ACE-536 Cmax and AUC after the first dose increased in a dose-proportional manner, with mean time to Cmax 7.7–11.7 days and mean elimination T½ 15.5–18.5 days.
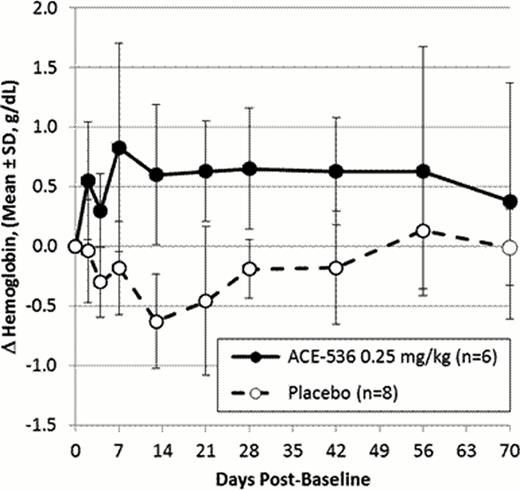
The maximum hemoglobin increase from baseline at any timepoint for subjects in the 0.25 mg/kg cohort ranged from 0.6 to 2.0 g/dL (5 of 6 subjects increased ≥1.1 g/dL), with a mean maximum increase of 1.3 g/dL (p<0.001 vs placebo). Mean hemoglobin levels increased in that dose group by at least 0.6 g/dL from Day 8 through Day 57, compared with a mean decrease in the placebo group of up to 0.6 g/dL through Day 43 (see Figure). Small increases in mean reticulocyte count and EPO levels were seen in groups treated with higher doses of ACE-536 as compared with the placebo group.
The preliminary results from this first-in-human phase 1 study show that ACE-536 is associated with a robust and sustained increase in hemoglobin levels in healthy subjects. Dose levels up to 0.25 mg/kg were generally safe and well-tolerated. The PK results support SC dosing of ACE-536 every 3 weeks. These data warrant further evaluation of ACE-536 in clinical trials in patients with disease-associated ineffective erythropoiesis and anemia, such as MDS and β-thalassemia.
Attie:Acceleron Pharma, Inc.: Employment. Boyd:Acceleron Pharma, Inc.: Employment. Wilson:Acceleron Pharma, Inc.: Employment. Sherman:Acceleron Pharma, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal