Abstract
Abstract 3193
Paroxysmal Nocturnal Hemoglobinuria (PNH) is an acquired hemolytic anemia characterized by intravascular hemolysis, which is effectively controlled with eculizumab. However, in some cases, unexplained upper limit lactate dehydrogenase levels (LDH), as well as low haptoglobin levels are not unusual under treatment, suggesting residual low-level hemolysis. C3-mediated clearance of PNH red blood cells has been recently suggested in patients (pts) under eculizumab (Risitano Blood 2010). We hypothesized residual hemolysis may also be due to incomplete C5 blockage under treatment. CH50 activitiy (residual functional C5 activity) as well as C3 deposition on PNH red blood cells were assessed among eculizumab-treated pts. We also examined the hypothesis that mutations in complement genes are implicated in residual hemolytic process.
From October 2010 to February 2012, 22 pts (7 male, median age 42 years range 21 to 72) with hemolytic PNH treated with eculizumab (900 mg intravenously every 14±2 days) were prospectively followed systematically every 2 weeks. Before each Eculizumab infusion, clinical data (abdominal pain, thrombosis events and transfusion requirements) as well as complete blood count, LDH, bilirubin levels and reticulocytes were systematically collected. Concomitantly, CH50 using sensibilized sheep red blood cells, C3d deposition on red blood cells using flow cytometry as well as C3 and C4 circulating levels were also studied. Complete C5 blockage was defined by a CH50 activity ≤10% (as for pts with hereditary complete C5 deficiency). All pts included in the study have at least 6 months follow-up under treatment. Only measurements performed after at least 6 months of treatment were considered. Residual intravascular hemolysis was defined by upper limit LDH level before eculizumab injection. CH50 was analyzed with a longitudinal tobit regression model accounting for repeated sampling and the limit of detection. The model was fit in a Bayesian framework, so no p-values are presented.
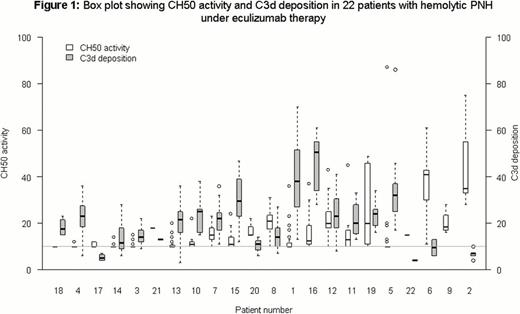
Before starting eculizumab, 21 pts were transfusion-dependent with a mean of 7 red blood cell (RBC) transfusions per year and 9 pts had a significant PNH-related complication (9 thrombosis and 7 with a previous history of aplastic anemia). During the study period, 6 pts received at least one transfusion (mean of 3 RBC transfusions per year) and 1 patient presented a deep vein thrombosis. All pts were analyzed for CH50 activity (412 samples; median 19 per patient; range, 4 to 31). Overall, CH50 measurements showed substantial variability for most pts (Figure 1). Residual CH50 activity (>10%) was significantly associated with higher LDH levels whereas pts who were still transfused (as well as pts with lower hemoglobin level and higher reticulocytes counts) tended to have higher CH50. Type III PNH red blood cell C3d deposition (assessed in all but 1 patient; 277 samples; median 14 per patient; range, 2 to 21) was found in all pts evaluated during the study period (Fig. 1). However, we did not find any correlation between C3 opsonization and clinical or biological signs of hemolysis. The association between CH50 and higher C3 deposition was weak (on average −0.8% CH50 per 10%) more C3 deposition (95%CI −1.7 to 0.2) (Fig. 1). Those results were confirmed in the subset of 15 patients with pure hemolytic PNH (no history of aplastic anemia). C3 and C4 circulating levels were in the normal range during the study period in all but one patient (pts#15, Fig. 1), who carry a complement factor H (CFH) mutation, leading to a quantitative Factor H deficiency. Systematic screening was positive for 2 other pts (CFH, pts#1; C3 mutation, pts#14) but with no phenotypical consequence.
Our results confirm the excellent overall clinical and biological response of hemolytic PNH pts to eculizumab. We found that unblocked CH50 activity, reflecting the residual C5 activity, was significantly associated with residual intravascular hemolysis while the selective C3d deposition of PNH red cells (found in all pts under treatment) was not. We also identified mutations in C3 and factor H genes. Whether incomplete C5 blockade is due to low residual level of eculizumab, complement mutations or polymorphisms, or other mechanisms are under investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal