Abstract
Multiple myeloma (MM) is an incurable cancer commonly treated with autologous stem cell transplantation (SCT). Response evaluation has traditionally been evaluated at 100 days after SCT, both to allow for hematopoietic reconstitution and due to the long half-life of immunoglobulins. Free light chains (FLC) are useful in the diagnosis, risk stratification, and treatment response assessment of plasma cell disorders. FLC have significantly shorter half-lives, and may provide evidence of response or treatment failure earlier after SCT; however their role in this setting has not been validated.
Patients were included in the study if they received intermediate or high dose chemotherapy (melphalan ≥ 100 mg/m2) with autologous stem cell support and had FLC measurements early after SCT, defined as 30 or 60 days after SCT.
Using the difference between involved and uninvolved FLC (dFLC), we established two criteria to identify patients at risk of early relapse:
dFLC rise: 10% increase in dFLC with an absolute increase of 25 mg/L
dFLC stable: in patients with pre-SCT dFLC greater than 100 mg/L, failure to reduce dFLC by 50%.
These criteria are less stringent than standard relapse criteria, as they were chosen to identify patients at risk of relapse before they meet full criteria for relapse. While there is an intra-patient variability in FLC that may argue for more stringent criteria, in the setting of high dose melphalan the natural course is rapid and deep reductions in FLC, which could allow smaller rises in FLC to have prognostic value.
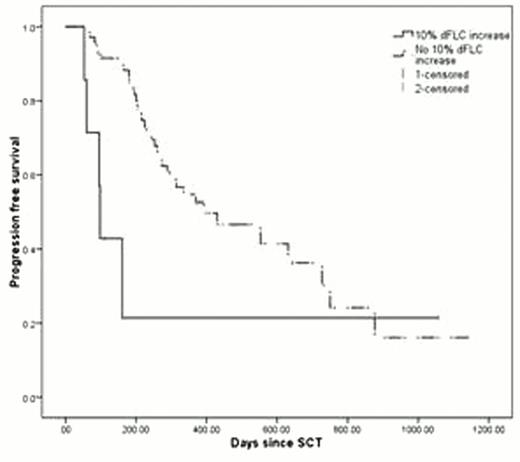
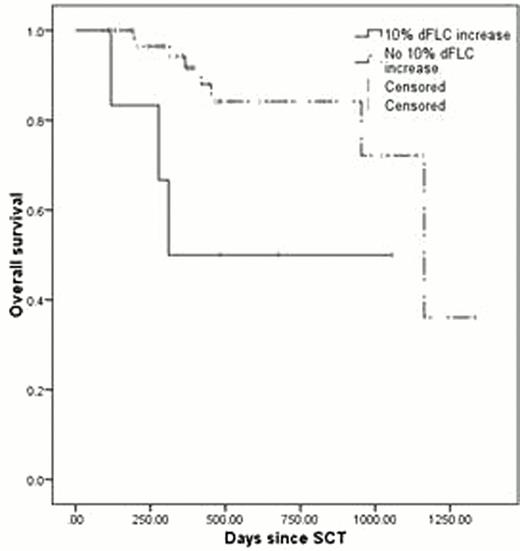
Eight-one patients met the inclusion criteria. Thirty-nine underwent SCT as part of the first line of therapy and the remaining 42 as a second line or higher therapy (median 3rd line). Patients with dFLC rise had shorter progression free (PFS; 98 vs. 393 days, p=0.04; Figure 1) and overall survival (OS; 10 versus 38 months, p=0.021; Figure 2). Patients meeting a composite of either high-risk criterion had shorter PFS, though not statistically significant (161 versus 430 days, p=0.192), whereas the OS was shorter (median not reached, p=0.009).
The majority of patients meeting the high-risk criteria were undergoing SCT as part of a 2nd or greater line of therapy. When analyzing this subset of patients, PFS was 96 days for those with dFLC rise compared to 229 days for standard risk patients (p<0.001), and OS was 310 days compared to 954 days (p=0.103). For patients meeting either high-risk criterion, PFS was 96 days compared to 244 days (p=0.013). Similarly, OS for these patients was 314 days compared to 954 days for normal risk patients (p=0.022).
To adjust for potentially confounding factors with known prognostic importance, we performed a multivariate cox regression with the covariates International Staging System stage, number of prior lines of therapy (0, 1–2, ≥3), and high-risk cytogenetic or FISH abnormalities. After adjusting for these covariates, early FLC assessment remained a significant prognostic factor for PFS and OS.
As newer treatments for myeloma provide superior responses, more patients are entering SCT with low or unmeasurable M-spikes. Due to their very short half-lives FLC are a potentially important prognostic factor after SCT. In our study, FLC assessment either one or two months post-SCT, particularly in those patients receiving SCT as second line or later regimen, was able to identify a subset of patients at high risk of early relapse. Our results offer the potential of early identification of patients who may benefit from early non-myelosuppressive or novel therapeutic interventions.
PFS for patients with 10% dFLC increase with an absolute increase of at least 25 mg/L.
PFS for patients with 10% dFLC increase with an absolute increase of at least 25 mg/L.
OS for patients with 10% dFLC increase with an absolute increase of at least 25 mg/L.
OS for patients with 10% dFLC increase with an absolute increase of at least 25 mg/L.
Jagannath:Onyx Pharmaceuticals, Inc.: Consultancy; Celgene Corporation: Scientific Advisory Board, Industry sponsored lecture, Scientific Advisory Board, Industry sponsored lecture Other; Millennium Pharmaceuticals, Inc. (Takeda Pharmaceutical Company Limited ): Scientific Advisory Board, Scientific Advisory Board Other; Merck & Co., Inc.: Industry Sponsored Lecture, Industry Sponsored Lecture Other; PharmaMar (Zeltia Group): Data Safety Monitoring Board Other. Chari:Array BioPharma: Scientific Advisory Board Other; Celgene Corporation: Scientific Advisory Board, Industry sponsored lectures, Scientific Advisory Board, Industry sponsored lectures Other; Onyx Pharmaceuticals, Inc: Scientific Advisory Board, Scientific Advisory Board Other.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal