Abstract
Outcome of aggressive HIV-associated NHL (HIV-NHL) with adverse prognostic features is not satisfactory with standard therapy. High dose therapy (HDT) and autologous stem cell transplantation (ASCT) has been demonstrated safe and active in HIV-NHL in the salvage setting.
To define the role of HDT and ASCT in the upfront treatment of HIV-NHL at high risk, in terms of efficacy and toxicity. We report the interim results of a multicenter study including HDT and ASCT as consolidation after first-line treatment of HIV-NHL at high-risk.
Inclusion criteria: untreated aggressive HIV-NHL (including DLBCL, plasmablastic, anaplastic lymphoma), aa-IPI 2–3, age 18–60, CD4+ count >50/mcl and availability of effective HAART. Burkitt and CNS lymphoma are excluded. Patients (pts) receive R-CHOP-21 (CHOP-21 or CHOP-14 for CD20 negative lymphoma) for 6 cycles and, if responsive, undergo stem cells mobilization and collection after Cyclophosphamide 4gr/ms + G-CSF followed by HDT with BEAM and ASCT. Involved-field radiotherapy on previous bulky or residual disease is recommended. Pts receive HAART during the entire treatment program. Overall survival (OS) at 2 years is the primary endpoint.
Since January 2007 to July 2012, 23 pts were registered and 20 entered the study. Median age was 47.5 years (range, 27–62). Fifteen pts (75%) had DLBCL, 4 (20%) plasmablastic and 1 (5%) anaplastic lymphoma. ECOG PS was >1 in 11 pts (55%); Ann-Arbor stage III/IV, 5(25%)/15(75%); B symptoms, 12 (60%); LDH > n.v., 18 (90%); aaIPI 2/3, 10 (50%)/10(50%). Fourteen pts (70%) had a prior history of HIV-positivity and 13 (65%) were on HAART at NHL diagnosis; thirteen (65%) had detectable HIV-viremia. Median CD4+ count was 283/mcl (58–571). Nine pts (45%) had HCV infection. Seventeen pts received (R)-CHOP-21 and 3 pts with plasmablastic histology received CHOP-14. One pt is still on treatment and 19/20 are evaluable for (R)-CHOP response. One had toxic death (due to hepatic failure), 2 had prolonged cytopenia (1 with severe hepatic toxicity) that lead to withdrawal of therapy and 16 completed (R)-CHOP therapy: 11 pts had complete remission (CR), 4 partial remission (PR) and 1 disease progression (PD) (ORR 79%,CR 58%, PR 21%, according to intention to treat). One pt is ongoing and 14 collected CD34+ cells after Cyclophosphamide + G-CSF (median CD34+ cells 7.35 × 10e6/Kg, range 2.65–10.0). Two pts had early disease progression after collection, 1 did not receive HDT because of cardiac ejection fraction < 50% at evaluation before BEAM and 1 is ongoing. So far, 10 pts received ASCT according to the protocol. Moreover, three pts received radiotherapy (2 on previous bulky and 1 on testicular disease).
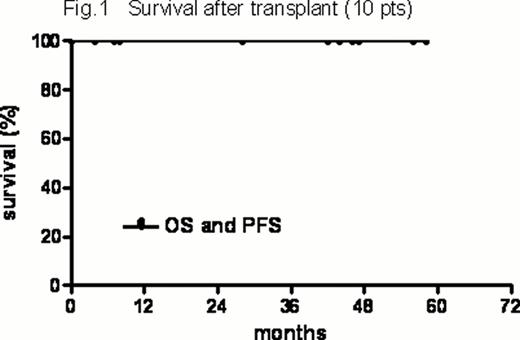
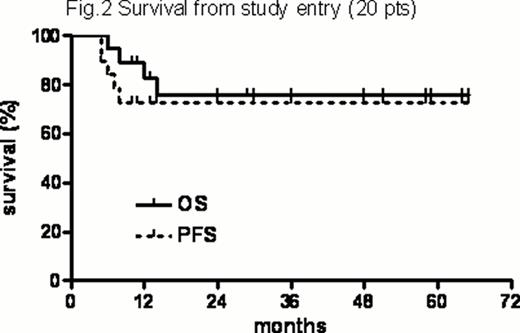
HDT-related toxicities included 2 grade II and 5 grade III GI and 1 grade III hepatic toxicity. Prior to engraftment 1 VZV infection, 1 Clostridium difficile colitis, 1 sigmoiditis and 5 episodes of FUO were registered. There were no transplant-related deaths. One case of CMV reactivation and no opportunistic infections were registered during observation. All transplanted pts are alive and relapse-free after a median of 43 ms (range, 4–58) after transplant (Figure 1). In the entire series, with a median f-up of 29.5 ms (range, 4–65), the 2-years PFS and OS of the entire series from study entry were 73% (+10.3%) and 76% (+10.6%), respectively (Figure 2).
This is the first prospective trial addressing the role of HDT and ASCT in first line treatment of HIV-LNH. The procedure was well tolerated and the clinical results highly encouraging. Interim evaluation of OS in this very high risk series of pts is satisfactory and accrual is ongoing.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal