Abstract
Abstract 3056
The pathogenesis of GVHD is not fully understood. Alloreactive T-lymphocytes are believed to be key mediators of GVHD. However, it is not clear if the pathobiology of GHVD is similar in each target organ GVHD. We aimed to identify predictive single nucleotide polymorphisms (SNP) markers associated with the risk of acute or chronic graft versus host disease (GVHD) as well as organ specific GVHD in 394 transplant recipients and donors.
A total of 259 SNPs were genotyped in 53 genes, and evaluated for the risk of acute/chronic GVHD and organ specific GVHD. Predictive models were generated using both clinical factors and genetic SNP markers confirmed by multivariate analyses. Patients were stratified by quartile (25%) according to their risk score, and the risk of overall and organ specific GVHD were compared among the 3 risk groups (low, intermediate and high risk). C-statistic analysis was also performed to compare the stratification power of the predictive model generated using clinical and genetic factors with a model obtained using only clinical factors.
Several SNP markers in the cytokine-, apoptosis-, TGF-¥â or PDGF-mediated pathways were identified as predictive markers of acute/chronic GVHD. The risk of acute GVHD was associated with clinical factors such as HLA disparity and patient age. In addition, recipient FAS genotype (rs2234978), EDN1 genotype (rs4714384), and TGFB genotype (rs1800469), and donor TNFRII genotype (rs3397) were also strong predictive markers for acute GVHD. Significant predictive risk factors forchronic GVHD were the source of stem cells, a previous episode of acute GVHD and the donor IL1R1 genotype (rs3917225).
Each organ specific GVHD shared common biologic pathways such as cytokine, TGF-¥â or PDGF-mediated pathways. However, different SNP markers were identified as predictive for individual organ-specific GVHD. Multivariate analyses identified several SNP markers may predict the risk of organ specific acute GVHD in combination with clinical factors. For skin acute GVHD, recipient PDGFD (rs10895534), donor NOS2A (rs3730017), TNFRII (rs3397) and TGFB1 (rs1800469) genotypes were predictive together with clinical factors such as HLA disparity. Donor's genotype for TNFRII (rs3397) was predictive not only for overall acute GVHD but also for skin acute GVHD. No clinical factors were identified for the risk of liver or gut acute GVHD, but several SNP markers were found including recipient PDGFRB (rs2302273), IFNGR1 (rs2234711) and donor PTGS1 (rs10306114), NOS1 (rs9658254), IL1R1 (rs2192752) genotypes for liver acute GVHD and recipient IL4 (rs2243248), donor PDGFD (rs1053861), TGFBR1 (rs420549), IL12A (rs2243115) genotypes for gut acute GVHD. In summary, there are no overlapping SNP markers for the risk prediction of organ specific acute GVHD.
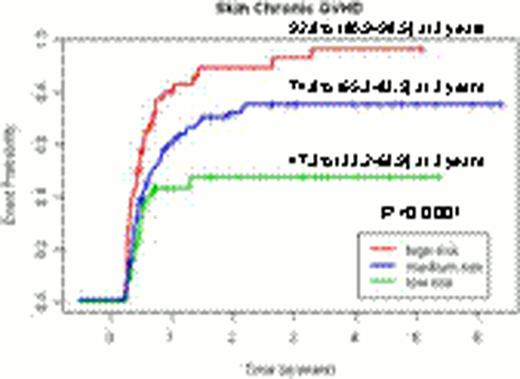
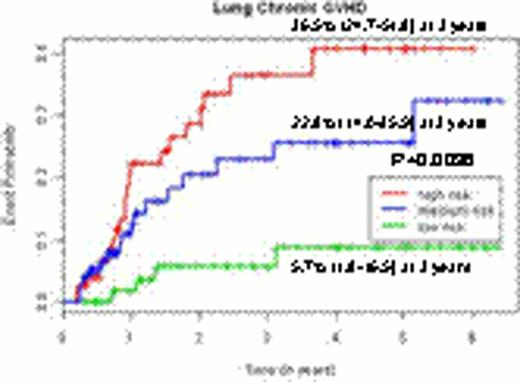
For organ specific chronic GVHD, 2 clinical risk factors were predictive including source of stem cells and a preceding history of acute GVHD. In addition, several SNP markers were also identified: recipient PDGFC (rs1425486), donor NFKB1 (rs1805034) and NOS2A (rs3730017) for skin chronic GVHD; recipient IL10RB (rs8178561) and PDGFRB (rs22229562), and donor TGFBR1 (rs868) for eye chronic GVHD; recipient IL12RB1 (rs3746190) and donor FCGR2A (rs1801274) for oral chronic GVHD; and donor IL4R (rs2057768), FAS (rs2234767) and TGFB1 (rs1800469) for lung chronic GVHD. Again, In no overlapping SNP markers were observed for organ-specific chronic GVHD risk.
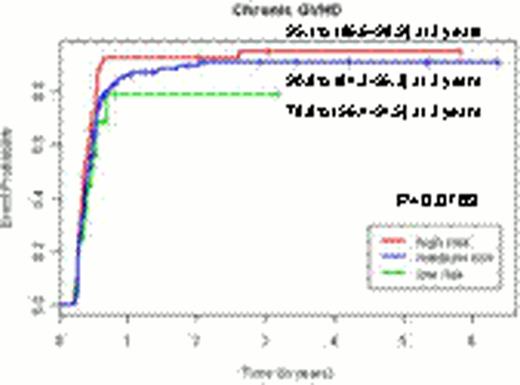
Although this predictive model could not stratify patients according to their risk of overall chronic GVHD (p=0.0763), the predictive models per each organ specific chronic GVHD enabled to stratify the patients according to their risks of each organ specific GVHD (p<0.0001 for skin chronic GVHD, p=0.0033 for eye chronic GVHD, p=0.003 for oral chronic GVHD and p=0.0036 for lung chronic GVHD).Predictive models incorporating clinical and genetic factors improved the stratification power by 11.1% compared to models only including clinical factors.
Our study suggests that SNP based approaches can predict the risk of organ-specific GVHD. These SNP markers need to be validated in other series. These SNPs may help focus studies into pathobiology and targeted therapy of GVHD in the future.
The incidence curves for chronic GVHD and organ-specific chronic GVHD according to the risk groups by the genetic predictive models.
The incidence curves for chronic GVHD and organ-specific chronic GVHD according to the risk groups by the genetic predictive models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal