Abstract
Abstract 2978
Novel agents have emerged as important therapeutic options for plasma cell dyscrasias and consequently have been adopted into treatment strategies for Systemic AL amyloidosis. Several studies to date have shown the efficacy of immunomodulatory drugs (IMiD) in this disease. However, little data exists exploring the efficacy of these agents especially in the context of previous treatment lines. The primary objective of this study was to evaluate the response, OS and PFS in patients treated with lenalidomide regimens in the relapsed setting. Further analysis was carried out examining outcomes based on previous lines of therapy. The importance of continuous therapy for control of the clonal disease stable disease was also examined.
Our primary cohort consisted of 66 AL amyloidosis patients who presented to our Centre between July 2007 and July 2012, identified from the database of the UK National Amyloidosis Centre, commencing a lenalidomide regimen in the relapsed setting. It was recommended that treatment continue until disease progression or unacceptable toxicity. Organ involvement and haematological and organ response were defined according to the 2010 consensus criteria. Overall survival (OS) was calculated from the start of lenalidomide treatment until death or last follow-up. Progression-free survival (PFS) was calculated from the start of lenalidomide treatment until relapse, death or last follow-up. Survival endpoints were examined based on previous lines of treatment. A subgroup analysis was carried out in those attaining at least stable disease to examine the impact of continuous therapy. In this cohort PFS was examined based on those remaining on continuous therapy versus those discontinuing treatment due to intolerance or patient preference.
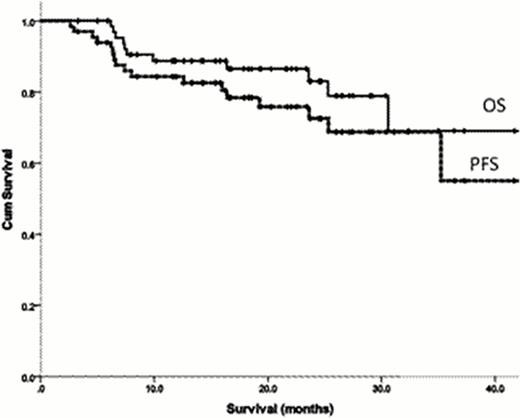
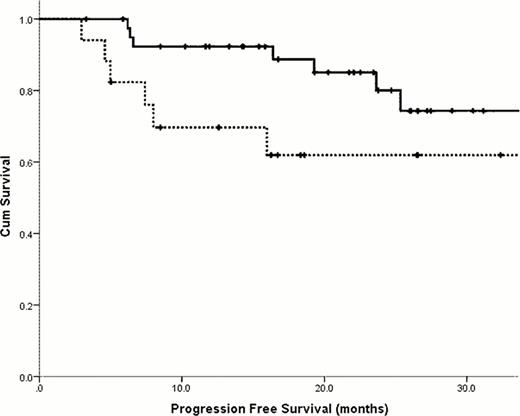
The median age was 59.1 years (range 39.9–76.9) and 52% were male. A lambda clonal plasma cell dyscrasia was present in 62%. 44% had ≥ 3 organ involvement with 50% having cardiac involvement by echo criteria. Mayo cardiac staging (at diagnosis) was stage I, II and III in 30%, 62% and 8% respectively. The median number of previous treatment lines was 2 (range 1–5), with thalidomide and bortezomib pre-treatment in 80% and 68% respectively. Grade 3 toxicity was seen in 27% of patients and was mainly haematologic. A thrombotic complication was noted in 1 patient. On an intention-to-treat basis, 56% achieved a haematological response with a complete response in 11%. A dFLC response was observed in 57% and 26% achieved a dFLC-VGPR or better. The median follow-up of 12.4 months. The median OS was 46.3 months and the estimated 2-year PFS was 67% (figure 1). Compared to those who did not receive the drug, previous exposure to thalidomide did not confer a worse survival outcome (estimated 2-year OS 67% vs 67%, p = 0.59). Similarly, previous exposure to Bortezomib did not confer a worse survival (estimated 2-year OS OS 88% vs 79%, p=0.09). 58 patients (88%) achieved stable disease or better. In this subgroup, the median duration on lenalidomide was 17.2 months. The 2-year OS and PFS were 84% and 78% respectively. Compared to those who halted therapy, continuous treatment with lenalidomide correlated with an improved PFS (estimated 2-year PFS 62% vs 80% respectively, p = 0.02).
This data further supports the effectiveness of lenalidomide containing regimens in the treatment of relapsed AL amyloidosis. Previous lines of therapy did not negatively impact survival outcomes. Continuous therapy is important in maximizing the duration of the clonal response.
Overall Survival (solid line) and Progression free survival (dashed line) for all patients treated with lenalidomide regimen at relapse.
Overall Survival (solid line) and Progression free survival (dashed line) for all patients treated with lenalidomide regimen at relapse.
Progression free survival comparing patients receiving continuous lenalidomide therapy (solid line) to those who discontinued treatment (dashed line).
Progression free survival comparing patients receiving continuous lenalidomide therapy (solid line) to those who discontinued treatment (dashed line).
No relevant conflicts of interest to declare.
References:
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal