Abstract
Abstract 2933
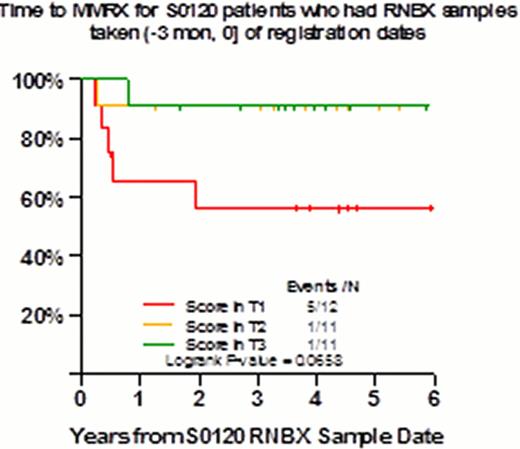
Monoclonal gammopathy of undetermined significance (MGUS), a premalignant plasma cell dyscrasia, is differentiated from asymptomatic multiple myeloma (AMM), based on the level of monoclonal immunoglobulin (M spike) and bone marrow plasma cell infiltration (M spike > 3 g/dl and/or marrow plasmacytosis >10% in AMM). MGUS progresses to active Multiple Myeloma (MM) at a rate of 1–2% per year, thus imparting an average risk of 25% of progression over a lifetime. No single clinical variable, imaging modality or molecular study has yet been identified to predict progression. As a part of SWOG observational trial S0120, 262 patients were enrolled at MIRT and their GEP of purified plasma cells (PC) prospectively obtained. Here we report the results of GEP as predictor of progression. Among those with baseline GEP data available, 127 were non-IgM, AMM or MGUS and were otherwise eligible for the study. Baseline samples were restricted to those samples taken no more than 6 months prior to or 14 days following registration to S0120. If a patient had multiple baseline samples, then the sample taken closest to the patient's registration date was used. GEP studies were performed to assign molecular classification and risk scores, along with GEP-derived amp1q21, delTP53, DKK1 and other variables. With a follow-up of 36 months in the subset with evaluable GEP baseline data in 40 MGUS and 87 AMM cases, GEP characteristics were compared with those of untreated MM patients treated with TT2, TT3, TT4, and TT5 protocols, in the context of normal donor PC signatures. Comparing MGUS with AMM, CD-2 molecular subgroup was over-represented in MGUS and HY in AMM. PSMD4 on chromosome 1q21 was also linked to AMM. In terms of GEP-70 risk model, 36% of AMM and 15% of MGUS had scores exceeding -0.26. Univariate predictors for treatment-requiring MM included the well-known serum-M and bone marrow PC variables, and GEP-70 score >-0.26 (HR=8.82, p<0.001). Whole bone marrow biopsy (BMB) GEP samples from MGUS/AMM were available in 26 samples. Applying a BMB-65 model distinguishing MM-BMB from NL-BMB (normal donors) and MGUS/AMM-BMB, we observed that there was a strong trend for delayed MM progression when MGUS/AMM-BMB scores were in the top tertile (most NL-like). Independent validation data will be presented.
Dhodapkar:Celgene: Research Funding; KHK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal