Abstract
Abstract 2788
Tyrosine kinase inhibitor (TKI) therapy has become the standard treatment for patients with chronic myelogenous leukemia (CML). It can induce durable hematologic, cytogenetic and molecular response, leading to a marked improvement of progression-free survival (PFS). On the other hand, long-term discontinuation of TKIs has recently been investigated by many groups. Our study was designed to confirm whether TKI could be safely discontinued in Japanese patients who have maintained complete molecular response (CMR) for at least 2 years, and to identify possible factors associated with prolonged drug-free survival (DFS), including immunologic profile. The effect of imatinib discontinuation in terms of quality of life (QOL) was also assessed.
Adult patients with CML who have sustained CMR (defined as negative quantitative and qualitative PCR of bcr-abl in the bone marrow) for more than 2 years were enrolled in the study. Treatment with imatinib or one of the other TKIs was initiated if the peripheral blood quantitative PCR (TMA method) value exceeded 100 copies. Lymphocyte subset analysis was performed before discontinuation of the drug, and at 6 months after discontinuation or re-induction of the drug in case of relapse. In 6 patients, WT-1 specific cytotoxic T lymphocyte (CTL) frequency was also assessed before, 3 and 6 months after drug discontinuation. QOL analysis was performed using SF-36 questionnaire before, 2 months and 1 year after discontinuation of imatinib.
41 patients were enrolled in the study, among which 40 patients were analyzed. The median age of the patients was 54 (range 28 – 83) years old. The Sokal risk score was low in 24 (60%), intermediate in 10 (25%) and high in 3 (7.5%) patients. The median time on imatinib treatment was 98 (range 24–126) months and the median duration of CMR was 49.5 months (range 24–106).
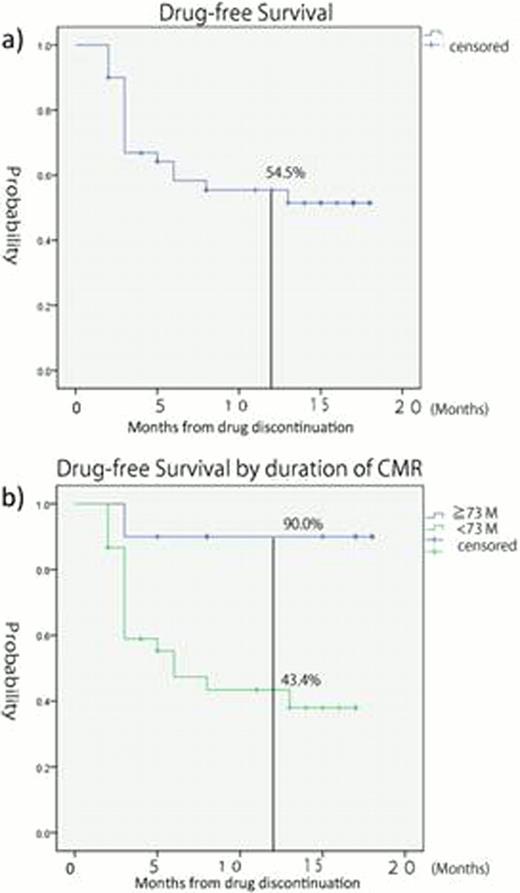
The median follow-up of the patients at the time of this analysis was 15.5 months (range 2–18). Treatment was restarted in 18 patients (45%), and the estimated DFS rate at 12 months was 55.4% (Fig 1). In 5 patients, imatinib was commenced again, whereas 13 patients were re-treated with dasatinib. All but one patient restored CMR after commencing TKIs.
Among various factors including age, previous interferon treatment, duration of imatinib treatment, duration of CMR, time until CMR, sex, cytomegalovirus serology and Sokal risk score, duration of CMR was identified as a significant factor associated with prolonged DFS on univariate analysis (p=0.027), the difference which was also significant upon multivariate analysis (p=0.014).
Regarding lymphocyte subsets in the peripheral blood, no significant changes were observed in CD4, 19, 56, ab TCR, gd TCR, CD4/CD25 positive cell population, but, there was a significant increase in the proportion of CD8 positive T cells among those who relapsed and those who did not (2.4% vs −2.4%, p=0.04). There was a trend for increased proportion of WT-1 specific CTL in patients who were restarted on TKI therapy.
QOL scores of both physical and mental domains did not differ significantly with the discontinuation of imatinib or re-initiation of treatment, although symptoms such as facial puffiness or muscle cramping were markedly decreased with discontinuation. There was also no difference in the patients' QOL according to the choice of drug used for re-treatment.
Altogether 6 patients had fluctuating PCR copy number during follow-up, of which 2 were restarted on treatment. Others have maintained low copy number or have returned to negative during follow-up. Due to the small number of patients, no specific clinical factors or immunophenotypes associated with sustained low count PCR were identified.
Sustained CMR was achieved in a substantial proportion of patients who had been in CMR for over 2 years. All patients restarted on TKI treatment remained sensitive to treatment. Longer time in CMR was identified as a significant factor related to sustained CMR in our patient population. Increase in CTL may also correlate with the necessity to restart treatment. Longer observation period and increased number of patients is necessary to draw a concrete conclusion, and to identify the role of immunologic profiles relative to persistence of CMR.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal