Abstract
Abstract 2785
Targeted therapy in CML with tyrosine kinase inhibitor (TKI) imatinib has resulted in significant improvement in outcome. However, resistance and intolerance to imatinib have seriously limited the success of this therapy, with only a proportion of patients achieving major molecular response (MMR). It is important to identify predictors of response to imatinib, since early switch to second generation TKI has been shown to improve outcome in non-responders. Overexpression of efflux transporters, decreased expression of influx transporters and polymorphisms in these transporters and drug metabolizing enzymes have been shown to influence imatinib therapy. We evaluated the role of RNA expression and polymorphisms in imatinib influx and efflux transporters in 86 newly diagnosed patients with CML at our centre receiving imatinib from December 2009 till April 2012. Total RNA was extracted and RNA expression of the transporter genes ABCA3 (sequesters imatinib; increased expression shown to decrease imatinib exposure), ABCB1, ABCG2 (both efflux transporters) and SLC22A1 (influx transporter) were analyzed with GAPDH as housekeeping gene using Taqman based gene expression assays. The dCT values (where dCT = CT value of target gene – CT value of housekeeping gene; lower dCT corresponds to higher expression and vice versa) were used for comparing expression between groups. Polymorphisms in ABCB1 (T1236C, T2677A, and C3435T), ABCG2 (Ex2 G34A and Ex5 C421A, promoter SNP -15994C->T), SLC22A1 (all coding exons) that were associated with the expression of these transporters were analyzed from genomic DNA samples by PCR followed by direct sequencing or RFLP. BCR-ABL transcript levels were monitored at diagnosis, 3, 6, 9, 12, 18, 24 and 30 months after start of imatinib. Molecular response to imatinib after a minimum of 12 months was calculated using the ratio of BCR-ABL to ABL transcript expressed in International scale. Patients were classified to have MMR (BCR-ABL/ABL <0.01) or no MMR (BCR-ABL/ABL >0.01; but complete or partial cytogenetic response [CCR or PCR] based on FISH analysis at around 12 months from the start of imatinib therapy), and resistant (BCR-ABL/ABL >10; no cytogenetic response even after imatinib dose was increased), or intolerant (severe cytopenia or skin toxicity requiring frequent dose reductions). The RNA expression was compared between different groups by ANOVA and the effect of SNPs on outcome was compared by Fisher's test.
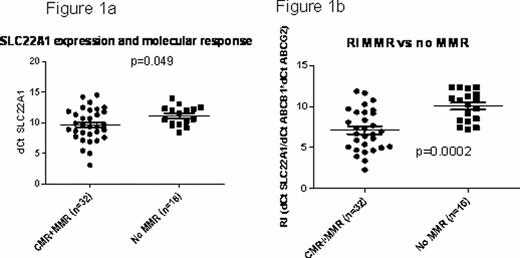
Out of the 86 patients, 34 achieved MMR, 22 did not achieve MMR; 14 were resistant and 12 were intolerant to imatinib; 4 patients progressed. RNA expression of ABCB1, ABCG2, ABCA3 and SLC22A1 showed wide variation among CML patients. RNA expression of SLC22A1 was significantly higher in those with MMR compared to no MMR; but not significantly different from resistance or intolerant groups (Fig 1a). ABCA3, ABCG2 and ABCB1 expression, though lower in the MMR group compared to no MMR, did not reach statistical significance. We then compared the ratio of influx to efflux transporters: dCThOCT1/dCTABCB1*dCTABCG2, referred to as resistance index (RI), in these groups; patients with MMR had significantly lower RI compared to those who did not achieve MMR (Fig 1b).
We then compared the influence of SNPs in candidate genes with molecular response to imatinib. There was significantly increased frequency of variant alleles of MDR1 C3435T and T1236C in those who achieved MMR compared to those who did not achieve MMR/ resistant (p=0.03 and 0.01 respectively). We identified nine coding nonsynonymous variants including a novel exon2 variant Gly-165-Cys and 2 intronic variants in SLC22A1 upon sequencing. Among these SNPs, Pro341Leu in exon6 (p=0.015) and –TGA del polymorphism resulting in frame shift in exon 7 (p value=0.02) were significantly associated with decreased molecular response. None of the screened SNPs were particularly different in the intolerant patients.
In conclusion, we show for the first time a predictive model with RNA expression pattern and genotype that could identify CML patients who would achieve MMR on imatinib therapy. The group that did not achieve MMR, but partial or complete cytogenetic response, could be made to achieve MMR by increasing the dose of imatinib or modulating the candidate ABC transporter expression. However, this model could not differentiate patients who develop therapy resistance or intolerance, which needs to be evaluated further.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal