Abstract
Abstract 2730
Recently, L-asparaginase-based combination chemotherapy was found to be effective in salvage treatment in patients with relapsed or refractory extranodal NK/T-cell lymphoma, nasal type. To explore the single-agent activity of L-asparaginase, we conducted a single-institute, prospective phase II study.
Patients with relapsed or refractory extranodal NK/T-cell lymphoma, nasal type were eligible for enrollment regardless of prior treatment. L-asparaginase monotherapy (6000 U/m2 on days 1 to 7) was administered as the protocol treatment and repeated every 3 weeks for at most 8 cycles. For responding patients, the decision to proceed with hematopoietic stem-cell transplantation was made at the discretion of treating physicians. The primary endpoint was the best objective response after L-asparaginase.
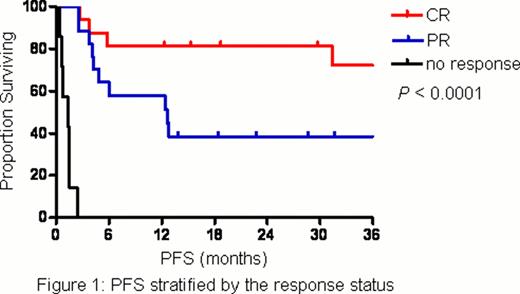
A total of 40 patients were enrolled and treated with L-asparaginase for a median of 5 cycles (range, 1 – 8). The patient characteristics were shown in Table 1. Half of the patients had stage IV disease at enrollment and the vast majority (18 patients) presented with disseminated cutaneous and soft-tissue involvement. Thirty-seven patients (92.5%) had prior exposure to systemic chemotherapy and 14 of them (37.8%) received more than 1 line. The overall response rate was 82.5%. The complete response (CR) and partial response (PR) rates were 40% and 42.5%, respectively. The incidence of adverse events was shown in Table 2. In short, anemia, neutropenia, hypoalbuminemia, nausea and liver-related disorders were common toxicities, which were usually mild and manageable. No grade 4 adverse events and treatment-related mortality were observed. Five patients (12.5%) developed allergic reaction to L-asparaginase and 3 of them had to withdraw from the study since L-asparaginase re-challenge with prophylactic antiallergic agents was unsuccessful. After a median follow-up time of 31.6 months (range, 21.9 – 41.3), the median progression-free survival (PFS) was 12.8 months and median overall survival (OS) was not reached. Response status (CR, PR or no response) after L-asparaginase had a significant impact on either PFS (Figure 1) or OS (Figure 2). Moreover, its prognostic value was confirmed in the multivariate analysis.
L-asparaginase demonstrated a high single-agent activity in salvage setting for patients with extranodal NK/T-cell lymphoma, nasal type. The first-line L-asparaginase-containing chemotherapy regimen warrants urgent investigation.
Patient Characteristics
| . | No. . | % . |
|---|---|---|
| Total number | 40 | 100 |
| Median age (years, range) | 51 (32–69) | |
| Gender | ||
| Male | 26 | 65 |
| Female | 14 | 35 |
| Performance status | ||
| 0 | 3 | 7.5 |
| 1 | 36 | 90 |
| 2 | 1 | 2.5 |
| B symptoms | ||
| Yes | 18 | 45 |
| No | 22 | 55 |
| Lactate dehydrogenase | ||
| Elevated | 13 | 32.5 |
| Normal | 27 | 67.5 |
| Primary site at first diagnosis | ||
| Upper aerodigestive tract | 31 | 77.5 |
| Non- upper aerodigestive tract | 9 | 22.5 |
| Stage at enrollment | ||
| IE | 12 | 30 |
| IIE | 7 | 17.5 |
| IIIE | 1 | 2.5 |
| IV | 20 | 50 |
| International Prognostic Index | ||
| 0–1 | 28 | 70 |
| 2 | 8 | 20 |
| 3 | 4 | 10 |
| Korean Prognostic Index | ||
| 0 | 4 | 10 |
| 1 | 18 | 45 |
| 2 | 13 | 32.5 |
| 3–4 | 5 | 12.5 |
| Prior treatment | ||
| Radiotherapy alone | 3 | 7.5 |
| Chemotherapy alone | 9 | 22.5 |
| Chemotherapy and radiotherapy | 28 | 70 |
| Disease status | ||
| Relapsed | 16 | 40 |
| Refractory | 24 | 60 |
| . | No. . | % . |
|---|---|---|
| Total number | 40 | 100 |
| Median age (years, range) | 51 (32–69) | |
| Gender | ||
| Male | 26 | 65 |
| Female | 14 | 35 |
| Performance status | ||
| 0 | 3 | 7.5 |
| 1 | 36 | 90 |
| 2 | 1 | 2.5 |
| B symptoms | ||
| Yes | 18 | 45 |
| No | 22 | 55 |
| Lactate dehydrogenase | ||
| Elevated | 13 | 32.5 |
| Normal | 27 | 67.5 |
| Primary site at first diagnosis | ||
| Upper aerodigestive tract | 31 | 77.5 |
| Non- upper aerodigestive tract | 9 | 22.5 |
| Stage at enrollment | ||
| IE | 12 | 30 |
| IIE | 7 | 17.5 |
| IIIE | 1 | 2.5 |
| IV | 20 | 50 |
| International Prognostic Index | ||
| 0–1 | 28 | 70 |
| 2 | 8 | 20 |
| 3 | 4 | 10 |
| Korean Prognostic Index | ||
| 0 | 4 | 10 |
| 1 | 18 | 45 |
| 2 | 13 | 32.5 |
| 3–4 | 5 | 12.5 |
| Prior treatment | ||
| Radiotherapy alone | 3 | 7.5 |
| Chemotherapy alone | 9 | 22.5 |
| Chemotherapy and radiotherapy | 28 | 70 |
| Disease status | ||
| Relapsed | 16 | 40 |
| Refractory | 24 | 60 |
Incidence of Adverse Events
| . | Any Grade (%) . | Grade 3 (%) . | Grade 4 (%) . |
|---|---|---|---|
| Hematologic | |||
| Anemia | 90 | 5 | 0 |
| Neutropenia | 75 | 15 | 0 |
| Thrombocytopenia | 7.5 | 0 | 0 |
| Nonhematologic | |||
| Allergic reaction | 12.5 | 0 | 0 |
| Hyperbilirubinemia | 32.5 | 0 | 0 |
| ALT elevation | 35 | 0 | 0 |
| AST elevation | 27.5 | 0 | 0 |
| AKP elevation | 30 | 0 | 0 |
| GGT elevation | 32.5 | 2.5 | 0 |
| Hypoalbuminemia | 85 | 0 | 0 |
| Hyperamylasemia | 7.5 | 0 | 0 |
| Hyperglycemia | 15 | 0 | 0 |
| Hyponatremia | 15 | 0 | 0 |
| Hypokalemia | 12.5 | 0 | 0 |
| Hypomagnesemia | 10 | 0 | 0 |
| Nausea | 50 | 0 | 0 |
| Vomiting | 17.5 | 0 | 0 |
| Diarrhea | 10 | 2.5 | 0 |
| Constipation | 5 | 0 | 0 |
| Mucositis | 5 | 0 | 0 |
| Headache | 2.5 | 0 | 0 |
| Sensory neuropathy | 2.5 | 0 | 0 |
| . | Any Grade (%) . | Grade 3 (%) . | Grade 4 (%) . |
|---|---|---|---|
| Hematologic | |||
| Anemia | 90 | 5 | 0 |
| Neutropenia | 75 | 15 | 0 |
| Thrombocytopenia | 7.5 | 0 | 0 |
| Nonhematologic | |||
| Allergic reaction | 12.5 | 0 | 0 |
| Hyperbilirubinemia | 32.5 | 0 | 0 |
| ALT elevation | 35 | 0 | 0 |
| AST elevation | 27.5 | 0 | 0 |
| AKP elevation | 30 | 0 | 0 |
| GGT elevation | 32.5 | 2.5 | 0 |
| Hypoalbuminemia | 85 | 0 | 0 |
| Hyperamylasemia | 7.5 | 0 | 0 |
| Hyperglycemia | 15 | 0 | 0 |
| Hyponatremia | 15 | 0 | 0 |
| Hypokalemia | 12.5 | 0 | 0 |
| Hypomagnesemia | 10 | 0 | 0 |
| Nausea | 50 | 0 | 0 |
| Vomiting | 17.5 | 0 | 0 |
| Diarrhea | 10 | 2.5 | 0 |
| Constipation | 5 | 0 | 0 |
| Mucositis | 5 | 0 | 0 |
| Headache | 2.5 | 0 | 0 |
| Sensory neuropathy | 2.5 | 0 | 0 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; AKP: alkaline phosphatase; GGT: gamma-glutamyl transpeptidase
Off Label Use: L-asparaginase, which was used in our study for NK/T-cell lymphoma, is approved to treat acute lymphocytic leukemia by US and Chinese FDA.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal